Abstract
Background
Previous studies showed the inconsistent effects of malnutrition contributing to the poor prognosis of stroke. This study aims to explore the effect of malnutrition on 3- month functional prognosis of stroke patients with different stroke severity assessed by the national institute of health stroke scale (NIHSS).
Methods
Patients with first-ever stroke were consecutively enrolled in a nationwide, multicenter, and prospective registry from March 2007 to January 2008. Nutritional status was assessed at admission. Malnutrition was defined by any abnormality of 6 nutrition indicators, including body mass index (BMI), mid-upper arm circumference (MUAC), triceps skinfold thickness (TSF), haemoglobin, albumin, and prealbumin. Stroke patients were classified into mild (NIHSS<8) and severe (NIHSS≥8) groups. Multivariable logistic regression was performed to assess the risk of poor functional prognosis (modified Rankin Scale (mRS) ≥3) at 3-month follow-up in the mild or severe patients with malnutrition at admission.
Results
A total of 755 patients with first-ever stroke were enrolled in the study. Multivariable analysis showed that malnutrition independently contributed to a higher risk of mRS 3-6 at 3-month for mild stroke patients [odds ratio (OR) 1.86, 95% confidence interval (CI) 1.04-3.34], but didn’t for severe stroke patients (OR 0.91, 95% CI 0.53-1.54) after adjusting for confounders including age, NIHSS, and infection et al.
Conclusion
After adjusting for the potential confounders, malnutrition was still an independent risk factor for 3-month poor functional prognosis in mild stroke patients. Further investigation may be needed to illustrate the effects of improving nutritional status on stroke patients.
Keywords: Acute stroke, malnutrition, prognosis, national institute of health stroke scale, functional outcome, registry
1. INTRODUCTION
Stroke is a major cause of death and disability worldwide. Approximately three-quarters of the global burden of stroke deaths (approximately 6.5 million per year) and associated disability-adjusted life years (approximately 113 million) occurred in low- and middle-income countries [1, 2]. Accurate prognosis prediction can be used to optimize care and the allocation of healthcare resources.
There were developing awareness about the predictive value of malnutrition on stroke outcome. Many studies have shown that malnutrition or being at risk of malnutrition contributes to poor clinical outcomes [3-8]whereas others did not [9-12]. The variation may be attributed to the heterogeneity of nutrition indexes, patients’ group and characteristics, stroke severity and so on. There aren’t specific and validated nutrition assessment tools for stroke patients and not one single nutrition tool could reflect the nutrition status adequately and predict clinical outcomes [13]. Stroke severity, indicated by the national institute of health stroke scale (NIHSS), to a larger extent, would determine the functional outcome of stroke [14-16] than nutritional status and other factors, and is usually referred for different stroke treatment strategies [17]. It is plausible to investigate the effects of malnutrition on the functional outcome in patients with different stroke severity.
To illuminate these issues, we present the data from the investigation of nutrition status in stroke study (INSIS) involving about 1087 imaging-confirmed strokes from eleven study sites across China. We aimed to assess the malnutrition in stroke patients with different stroke severity at admission and investigate its potential role as a predictor of poor functional prognosis.
2. METHODS
2.1. Study Design and Participants
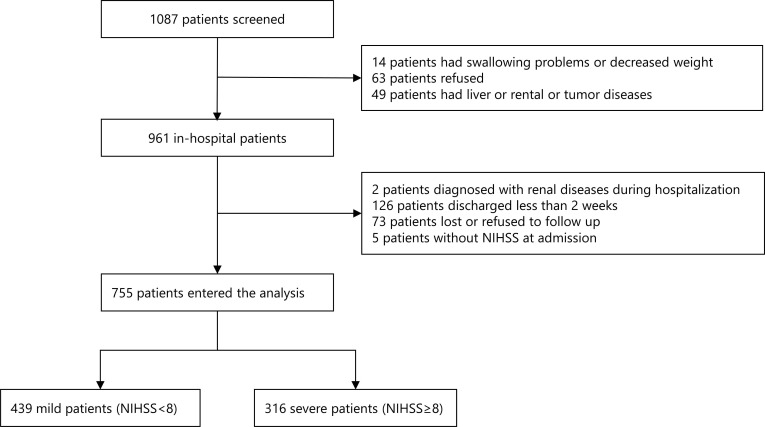
The design of this cohort study has been described in detail previously [18]. Generally, this was a nationwide, prospective, multicenter cohort registry designed to investigate nutrition status in stroke patients in China. This registry recruited consecutive patients from 11 centers across China if they were 18 years or older, within 7 days after the first stroke onset confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). From March 5, 2007 to January 31, 2008 in China, a total of 1087 patients were approached to participate in the study. Finally, 755 patients with stroke were divided into 439 mild stroke patients (NIHSS<8) and 316 severe (NIHSS≥8) ones (Fig. 1). This project was approved by the ethics committee at Beijing Tiantan Hospital and all participating centers.
Fig. (1).
Screening flowchart for stroke patients with and without malnutrition. A total of 1087 patients were screened. First, 126 patients were excluded due to swallowing problems or decreased weight existed before stroke, refusing to be recruited, and medical history of liver, renal or tumor diseases. Second, 206 patients were excluded because of the diagnosis of renal diseases during hospitalization, discharged less than 2 weeks, lost or refused to follow up and without NIHSS at admission. Finally, 755 patients with stroke, including 439 mild patients and 316 severe patients were included in this study for analysis. Aberrations: NIHSS, national institute of health stroke scale.
2.2. Data Collection
Professional research coordinators at each site center collected baseline data through a direct interview or medical record, including age, sex, health insurance, education, cigarette smoking, alcohol, medical history of digestive tract disease, high blood pressure, diabetes mellitus, coronary heart disease, atrial fibrillation, consciousness status, complication of infection and digestive tract ulcer. Blood samples were collected on the second working day after hospitalization and tested at each site center.
2.3. Nutritional Status Measurements
Nutritional status was assessed at admission (within 72 hours after admission), including body mass index (BMI) (weight in kilograms divided by the square of height in meters), mid-upper arm circumference (MUAC), triceps skinfold thickness (TSF), albumin, prealbumin, and haemoglobin. The abnormal criteria were as follows: BMI <18.5 kg/m2, MUAC below the tenth percentile of the reference value for Chinese people (males <24.8 cm and females <23.2 cm), TSF below the tenth percentile of the reference value for Chinese people (males <7.47 mm and females <13.8 mm), low haemoglobin count (males <120 g/L and females <110 g/L), serum albumin levels <35 g/L, and low serum prealbumin levels that were less than each hospital’s established criteria [18].
2.4. Follow-up and Outcomes
The detailed follow-up procedure has been previously described [18]. In brief, participants were followed up by face- to-face or telephone interview at 3 months by professional research personnel, collecting information of any death, stroke recurrence and cardiovascular events, and assessing patient’s the modified Rankin Scale (mRS) score ranging from 0 (no symptoms) to 6 (death) during the follow-up period.
2.5. Statistical Analysis
Categorical variables were presented as percentages and continuous variables were summarized as mean and standard deviation. Baseline characteristics were analyzed by chi-square statistics for the categorical variables and the Kruskal-Wallis test for the continuous variables. Patients were categorized into the mild group (NIHSS < 8) or severe group (NIHSS≥8). The predictive value of malnutrition for 3-month mRS (3-6) was analyzed by logistic regression with gradual models adjusting potential confounders. The unadjusted and adjusted odds ratios (ORs) and their 95 % confidence intervals (CIs) were calculated. A 2-sided P value of <0.05 was considered to indicate statistical significance. All data were analyzed by statistical software of SAS version 9.4 (SAS Institute Inc, Cary, NC).
3. RESULTS
Baseline characteristics, nutritional indicators of patients with different stroke severity, and nutrition status were shown in Table 1. Whether in the mild or severe stroke group, patients with malnutrition were older, more likely to be female, less educated, less likely to smoke, lower NIHSS at admission, and more likely to be atrial fibrillation. However, there was little difference in health insurance, history of alcohol, digestive tract ulcer, diabetes mellitus, unconsciousness, the value of blood urea nitrogen (BUN,) creatinine (Cr) and blood glucose with different nutrition statuses. Patients with malnutrition were more susceptible to have coronary heart disease(p<0.0001) and infections(p=0.0147) in severe patients but not in mild patients. In mild stroke patients, all nutrition indicators were lower in the malnutrition group than in the normal nutrition status group. In severe stroke patients, there was no difference in TSF between different nutrition status (p=0.1358), while the other 5 indicators were lower in the malnutrition group (Table 1).
Table 1.
Baseline characteristics of patients with normal nutrition or malnutrition at admission.
| Total | Mild | Severe | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Malnutrition | P value | Normal | Malnutrition | P value | |||
| NIHSS (Mean, SD) | 755 (7.8±6.5) | 324 (3.2 ±2.0) | 115 (3.9 ±2.2) | 0.0009 | 190 (13.3 ±4.9) | 126 (14.9 ±5.9) | 0.0123 | |
| Age (Mean, SD) | 755 (63.4 ±12.7) | 324 (61.1 ±11.8) | 115 (65.5±13.0) | 0.0009 | 190 (61.4 ±13.5) | 126 (70.2±11.1) | <0.0001 | |
| Female (%) | 265 (35.1 %) | 84 (25.9 %) | 50 (43.5 %) | 0.0006 | 62 (32.6 %) | 69 (54.8 %) | 0.0001 | |
| Health insurance (%) | 682 (90.3) | 308 (95.1 %) | 104 (90.4 %) | 0.1106 | 157 (82.6 %) | 113 (89.7 %) | 0.1030 | |
| Education (%) | 547 (72.5) | 262 (80.9 %) | 80 (69.6 %) | 0.0180 | 137 (72.1 %) | 68 (54.0 %) | 0.0011 | |
| Smoking (%) | 307 (40.7) | 153 (47.2 %) | 39 (33.9 %) | 0.0159 | 81 (42.6 %) | 34 (27.0 %) | 0.0059 | |
| Alcohol (%) | 132 (17.5) | 61 (18.8 %) | 16 (13.9 %) | 0.2565 | 37 (19.5 %) | 18 (14.3 %) | 0.2890 | |
| Prior disease history (%) | ||||||||
| Hypertension | 585 (77.5) | 260 (80.3 %) | 84 (73.0 %) | 0.1148 | 150 (79.0 %) | 91 (72.2 %) | 0.1790 | |
| Lipid abnormality | 433 (57.4) | 196 (60.5 %) | 62 (53.9 %) | 0.2268 | 118 (62.1 %) | 57 (45.2 %) | 0.0038 | |
| Digestive tract disease (%) | 54 (7.2) | 27 (8.3 %) | 7 (6.1 %) | 0.5446 | 10 (5.3 %) | 10 (7.9 %) | 0.3542 | |
| Coronary heart disease | 163 (21.6) | 66 (20.4 %) | 25 (21.7 %) | 0.7892 | 22 (11.6) | 50 (39.7) | <0.0001 | |
| Atrial fibrillation | 71 (9.4) | 13 (4.0 %) | 14 (12.2 %) | 0.0053 | 19 (10.0 %) | 25 (19.8 %) | 0.0194 | |
| Diabetes Mellitus (%) | 239 (31.7) | 107 (32.0 %) | 32 (27.8 %) | 0.3509 | 55 (29.0 %) | 45 (35.1 %) | 0.2184 | |
| Status on admission | ||||||||
| Unconsciousness (%) | 130 (17.3) | 8 (2.5 %) | 6 (5.3 %) | 0.2114 | 70 (36.8 %) | 46 (36.8 %) | 1.0000 | |
| Infections (%) | 59 (7.8) | 8 (2.5 %) | 5 (4.4 %) | 0.3391 | 20 (10.5 %) | 26 (20.6 %) | 0.0147 | |
| Digestive tract ulcer(%) | 16 (2.1) | 5 (1.6 %) | 2 (1.7 %) | 1.0000 | 7 (3.7 %) | 2 (1.6 %) | 0.3253 | |
| BUN(mmol/L) (Mean, SD) | 753 (5.6±3.5) | 323 (5.4 ±3.3) | 114 (5.5 ±4.1) | 0.9818 | 190 (5.7 ±2.2) | 126 (6.5 ±4.9) | 0.2934 | |
| Cr (μmol/L) (Mean, SD) | 753 (81.9±32.6) | 323 (81.3±20.1) | 114 (83.1±28.6) | 0.7620 | 190 (78.9 ±25.8) | 126 (87.2 ±59.7) | 0.6158 | |
| BG(mmol/L) (Mean, SD) | 746 (6.6±3.4) | 319 (6.3 ±2.5) | 114 (6.5±2.6) | 0.1245 | 188 (7.0 ±4.9) | 125 (6.9 ±2.8) | 0.7712 | |
| Nutrition indicators | ||||||||
| BMI (Kg/m2) (%) | 733 (24.7±3.6) | 310 (25.1±3.1) | 113 (23.6 ±3.8) | <0.0001 | 184 (25.3 ±3.5) | 126 (23.7 ±4.2) | <0.0001 | |
| MUAC (cm) (Mean, SD) | 715 (28.8 ±3.3) | 301 (29.7 ±2.6) | 115 (26.9 ±3.6) | <0.0001 | 174 (29.7 ±3.2) | 125 (27.2 ±3.4) | <0.0001 | |
| TSF (mm) (Mean, SD) | 715 (17.9±7.6) | 301 (18.2 ±6.9) | 115 (14.2 ±7.7) | <0.0001 | 174 (19.8 ±7.3) | 125 (18,2 ±8.3) | 0.1358 | |
| Haemoglobin (g/L) (Mean, SD) | 754 (140.1±18.3) | 323 (144.4±15.7) | 115 (131.5±18.5) | <0.0001 | 190 (145.2 ±16.1) | 126 (128.9 ±19.7) | <0.0001 | |
| Albumin(g/L) (Mean, SD) | 752 (40.6 ±4.3) | 322 (41.7±3.4) | 115 (38.9 ±5.4) | <0.0001 | 189 (41.6 ±3.5) | 126 (37.8 ±4.6) | <0.0001 | |
| Prealbumin(g/L) (%) | 719 (260.2±76.6) | 296 (284.3±72.1) | 115 (260.1±94.0) | <0.0001 | 183 (258.6 ±64.8) | 123 (206.9 ±56.7) | <0.0001 | |
Aberrations: BG, blood glucose; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; MUAC, mid-upper arm circumference; TSF, triceps skinfold thickness; NIHSS, national institute of health stroke scale.
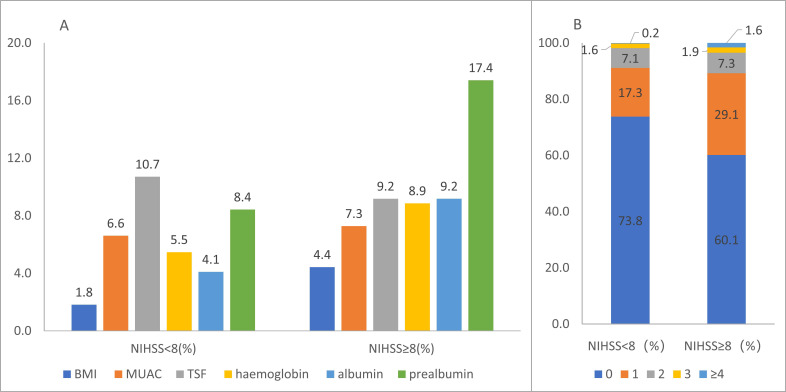
The abnormal rate of each indicator varied both in the mild group and in the severe group (Fig. 2A). A total of 26.2 % of stroke patients had one or more abnormal nutrition indicators in the mild group and 39.9 % in the severe group. Among 241 patients with abnormal nutrition indicators, about 70 % had one indicator abnormality. Approximately 1.8 % of mild patients and 3.5 % of severe patients had three or more abnormal indicators (Fig. 2B).
Fig. (2).
Nutrition status on admission in patients with stroke. Abnormal rate per nutrition indicator (A); percentage of abnormal nutritional indicators (B). The abnormal rate of each indicator ranged from 1.8 % to 10.7 % in the mild group and from 4.4 % to 17.4 % in the severe group. A total of 26.2 % of stroke patients had one or more abnormal nutrition indicators in the mild group and 39.9 % in the severe group. 0: without any abnormal nutritional indicators; 1: with one abnormal nutritional indicator; 2: with 2 abnormal nutritional indicators; 3: with 3 abnormal indicators; 4: with 4 or more abnormal nutritional indicators. Aberrations: BMI, body mass index; MUAC, mid-upper arm circumference; TSF, triceps skinfold thickness; NIHSS, national institute of health stroke scale (A higher resolution / colour version of this figure is available in the electronic copy of the article).
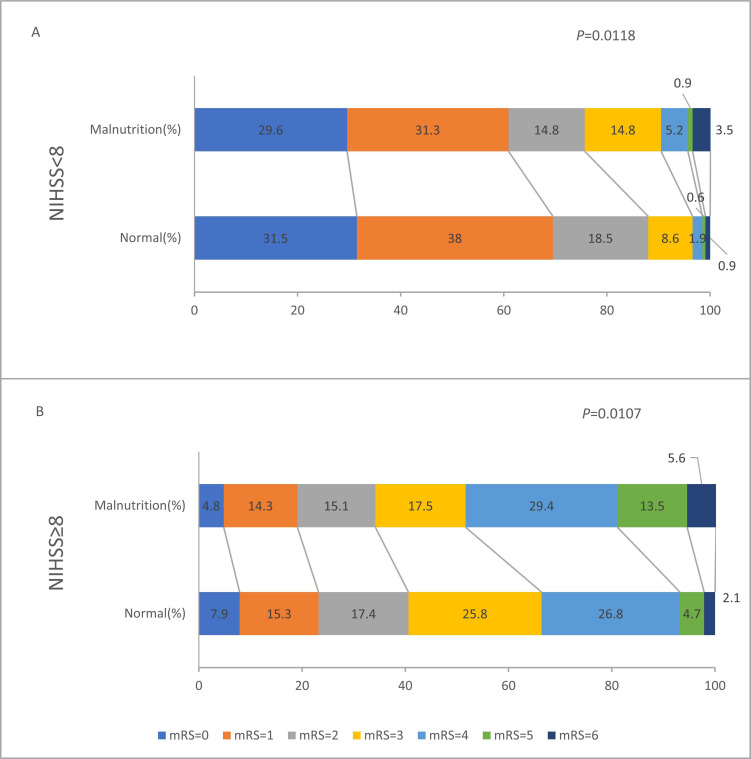
Patients with malnutrition had a greater unfavorable shift of mRS score distribution both in the mild group and in the severe group (Fig. 3).
Fig. (3).
Comparison of score distribution of mRS at 3-month after onset in the mild group (A) and severe group (B). Patients with malnutrition had a worse shift of mRS score distribution both in the mild group (unadjusted P=0.0118) and in the severe group (unadjusted P=0.0107). Aberrations: mRS, modified Rankin score; NIHSS, national institute of health stroke scale (A higher resolution / colour version of this figure is available in the electronic copy of the article).
After adjusting potential confounders of poor functional outcome, multivariable logistic regression analysis showed that malnutrition at admission was an independent factor for 3-month mRS 3-6 in mild patients but not in severe stroke patients (Table 2). The unadjusted and adjusted odds ratio (OR) of malnutrition in mild and severe patients for mRS 3-6 at 3-month was shown in Table 2.
Table 2.
The relationship of nutrition status and prognosis at 3-month after the onset of stroke by NIHSS.
| - | - | No. of Patients (%) | Unadjusted OR | Adjusted OR (Model 1) |
Adjusted OR (Model 2) |
Adjusted OR (Model 3) |
|---|---|---|---|---|---|---|
| NIHSS<8 | Normal | 39 (12.0) | Reference | |||
| Malnutrition | 28 (24.4) | 2.35 (1.37-4.04) | 1.92 (1.09-3.38) | 1.89 (1.07-3.35) | 1.86 (1.04-3.34) | |
| NIHSS≥8 | Normal | 112 (59.3) | Reference | |||
| Malnutrition | 84 (66.1) | 1.32 (0.82-2.10) | 1.01 (0.61-1.68) | 0.99 (0.59-1.65) | 0.91 (0.53-1.54) | |
Model 1: adjusted for age; gender.
Model 2: adjusted for age; gender; health insurance; education; history of smoking and alcohol.
Model 3: adjusted for age; gender; health insurance; education; history of smoking, alcohol and hypertension, diabetes mellitus, lipid abnormalities, coronary heart disease, atrial fibrillation, digestive tract disease, infections, digestive tract ulcer and NIHSS on admission.
4. DISCUSSION
On a large scale, nationwide stroke registry in China, we found that malnutrition at admission was an independent risk factor for functional disability of mild stroke patients. Our study discloses that functional outcomes in mild stroke patients are more susceptible to malnutrition than in severe patients, providing a new insight into understanding the association of malnutrition and stroke prognosis. Unlike age and stroke severity, malnutrition is a potentially modifiable factor that may improve stroke outcomes. It emphasizes that nutrition support should be further investigated as an important management strategy for improving the functional prognosis of the stroke patients, especially mild stroke patients.
Most studies investigating the prevalence and prognostic significance of malnutrition in stroke focused on patients with mild to moderate [19, 20]. Patients with severe stroke are more susceptible to undernourishment compared with those with mild strokes [21]. However, a study recruiting more than 600 severe stroke patients shows that malnutrition does not provide additional prognostic information concerning the risk of functional outcomes [11]. In our analysis, we provide additional evidence that malnutrition is a predictive risk factor for poor prognosis in mild patients, but not for severe patients. This can be used to identify those who might be more likely to benefit from nutritional support and develop individual nutrition care plans.
The possible explanation of our results may be that severe stroke indicated on NIHSS was a more determining factor on stroke functional prognosis than others [14-16], which may partially overpower the effects of malnutrition. Although nutritional status may deteriorate the muscle power of patients and potentially functional outcome, NIHSS is a decisive role in the functional outcome. Another factor may be the smaller sample of severe stroke in our study.
Poor nutrition has been associated with reduced muscle strength in patients [22, 23]. Muscle strength complications could hinder patients’ recovery and rehabilitation, resulting in poor functional prognosis and death. Low muscle mass at the onset of ischemic stroke is an independent predictor of walking function at discharge during the acute phase [24] and mRS at discharge [25]. In addition, malnutrition patients have a higher frequency of complications, including infection rates, gastrointestinal bleeding, and bedsores [3, 26, 27], which also could result in a poor prognosis.
The evaluation criteria of nutritional status are various [28], including simple assessments of weight and height, anthropometric measures, laboratory parameters, and more specialized measures. Prescribing doctors in the neurology department had little knowledge about nutritional risk index and mini nutritional assessment [29]. We have adopted 3 anthropometric indicators and 3 laboratory indicators, all of which are convenient to collect and widely used in routine clinical practice.
This study has some limitations. First, it is an observational study, so it fails to shed light on the causal relationship between malnutrition and poor prognosis. A double-blind, single-center study has reported that intensive nutritional supplementation improves motor recovery in previously malnutrition patients receiving intensive in-patient rehabilitation after stroke [30]. Therefore, further investigations of interventional programs in large-scale, multicenter studies are required to confirm the prognostic role of malnutrition in stroke care. Second, as blood protein compartments, haemoglobin [31], albumin and prealbumin [32] have been reported to be used as markers of nutritional status. However, they have also been criticized as players in nutritional assessment because of the lack of specificity, which was influenced by many complications, especially infections [33]. In our analysis, the relation between malnutrition and stroke prognosis is analyzed after adjusting for many confounding factors, including infections. Albumin was a good predictor of muscle dysfunction [34]. Nutritional indicators, especially some specific and measurable blood nutritional markers, may be novel intervention targets for patients with mild stroke. It is imperative to validate the effect of improving nutritional indicators by a standardized implementation strategy compatible with institutional practices and needs.
CONCLUSION
In conclusion, this study shows that baseline malnutrition independently predicts poor clinical outcomes in mild stroke patients. As a potentially modifiable risk factor for poor functional outcomes of acute stroke, more attention is needed to improve nutritional status in stroke care.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- BG
Blood Glucose
- BMI
Body Mass Index
- CI
Confidence Interval
- CT
Computed Tomography
- INSIS
Investigation of Nutrition Status In Stroke
- NIHSS
National Institute of Health Stroke Scale
- MRI
Magnetic Resonance Imaging
- mRS
Modified Rankin Scale
- MUAC
Mid-Upper Arm Circumference
- OR
Odds Ratio
- TSF
Triceps Skinfold Thickness
AUTHORS' CONTRIBUTORS
Zhang Jing designed the study. Zhang Jing and Qin Haiqiang conceived of and drafted the article, with further contributions from Yaqing Zhang. Bo Yang and Wei Na collected data. Wang Anxin and Zuo Yingting managed and analyzed the data. All authors interpreted the data and approved the final version of the article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee at Beijing Tiantan Hospital China with the project Number: 2006BAI01A11.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are base of this research. All the humans used were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics. iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Written informed consent was received from the patients.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available on request from the corresponding author. [J.Z], upon author request.
FUNDING
This work was financially supported by the National key R & D Plan of the Ministry of Science and Technology of China (Grant number 2016YFC1301604); National Natural Science Foundation of China (Grant number 81870907). National 11th &12th Five-year S &T Major Project (2006BAI01A11, 2011BAI08B01, 2011BAI08B02).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Feigin V.L., Roth G.A., Naghavi M., Parmar P., Krishnamurthi R., Chugh S., Mensah G.A., Norrving B., Shiue I., Ng M., Estep K., Cercy K., Murray C.J.L., Forouzanfar M.H. Global burden of diseases, injuries and risk factors study 2013 and stroke experts writing group. Global burden of stroke and risk factors in 188 countries, during 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016;15(9):913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 2.Feigin V.L., Krishnamurthi R.V., Parmar P., Norrving B., Mensah G.A., Bennett D.A., Barker-Collo S., Moran A.E., Sacco R.L., Truelsen T., Davis S., Pandian J.D., Naghavi M., Forouzanfar M.H., Nguyen G., Johnson C.O., Vos T., Meretoja A., Murray C.J., Roth G.A. GBD 2013 Writing Group; GBD 2013 Stroke Panel Experts Group. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: The GBD 2013 study. Neuroepidemiology. 2015;45(3):161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaboration F.T. FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: Observational data from the food trial. Stroke. 2003;34(6):1450–1456. doi: 10.1161/01.STR.0000074037.49197.8C. [DOI] [PubMed] [Google Scholar]
- 4.Cai Z.M., Wu Y.Z., Chen H.M., Feng R.Q., Liao C.W., Ye S.L., Liu Z.P., Zhang M.M., Zhu B.L. Being at risk of malnutrition predicts poor outcomes at 3 months in acute ischemic stroke patients. Eur. J. Clin. Nutr. 2020;74(5):796–805. doi: 10.1038/s41430-020-0605-8. [DOI] [PubMed] [Google Scholar]
- 5.Kokura Y., Maeda K., Wakabayashi H., Nishioka S., Higashi S. High nutritional-related risk on admission predicts less improvement of functional independence measure in geriatric stroke patients: A retrospective cohort study. J. Stroke Cerebrovasc. Dis. 2016;25(6):1335–1341. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Aliasghari F., Izadi A., Khalili M., Farhoudi M., Ahmadiyan S., Deljavan R. Impact of premorbid malnutrition and dysphagia on ischemic stroke outcome in elderly patients: A community-based study. J. Am. Coll. Nutr. 2019;38(4):318–326. doi: 10.1080/07315724.2018.1510348. [DOI] [PubMed] [Google Scholar]
- 7.Naito H., Nezu T., Hosomi N., Aoki S., Kinoshita N., Kuga J., Shimomura R., Araki M., Ueno H., Ochi K., Maruyama H. Controlling nutritional status score for predicting 3-mo functional outcome in acute ischemic stroke. Nutrition. 2018;55-56:1–6. doi: 10.1016/j.nut.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Nozoe M., Yamamoto M., Masuya R., Inoue T., Kubo H., Shimada S. Prevalence of malnutrition diagnosed with GLIM criteria and association with activities of daily living in patients with acute stroke. J. Stroke Cerebrovasc. Dis. 2021;30(9):105989. doi: 10.1016/j.jstrokecerebrovasdis.2021.105989. [DOI] [PubMed] [Google Scholar]
- 9.Martineau J., Bauer J.D., Isenring E., Cohen S. Malnutrition determined by the patient-generated subjective global assessment is associated with poor outcomes in acute stroke patients. Clin. Nutr. 2005;24(6):1073–1077. doi: 10.1016/j.clnu.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Davis J.P., Wong A.A., Schluter P.J., Henderson R.D., O’Sullivan J.D., Read S.J. Impact of premorbid undernutrition on outcome in stroke patients. Stroke. 2004;35(8):1930–1934. doi: 10.1161/01.STR.0000135227.10451.c9. [DOI] [PubMed] [Google Scholar]
- 11.Scrutinio D., Lanzillo B., Guida P., Passantino A., Spaccavento S., Battista P. Association between malnutrition and outcomes in patients with severe ischemic stroke undergoing rehabilitation. Arch. Phys. Med. Rehabil. 2020;101(5):852–860. doi: 10.1016/j.apmr.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Gomes F., Emery P.W., Weekes C.E. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J. Stroke Cerebrovasc. Dis. 2016;25(4):799–806. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 13.van Bokhorst-de van der Schueren M.A., Guaitoli P.R., Jansma E.P., de Vet H.C. Nutrition screening tools: Does one size fit all? a systematic review of screening tools for the hospital setting. Clin. Nutr. 2014;33(1):39–58. doi: 10.1016/j.clnu.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Rost N.S., Bottle A., Lee J.M., Randall M., Middleton S., Shaw L., Thijs V., Rinkel G.J., Hemmen T.M. Global Comparators Stroke GOAL collaborators. Stroke severity is a crucial predictor of outcome: An international prospective validation study. J. Am. Heart Assoc. 2016;5(1):e002433. doi: 10.1161/JAHA.115.002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozyolkin O., Kuznietsov A., Novikova L. Prediction of the lethal outcome of acute recurrent cerebral ischemic hemispheric stroke. Medicina (Kaunas) 2019;55(6):E311. doi: 10.3390/medicina55060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S., Toyoda K., Uehara T., Toratani N., Yokota C., Moriwaki H., Naritomi H., Minematsu K. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70(24 Pt 2):2371–2377. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 17.Fischer U., Arnold M., Nedeltchev K., Brekenfeld C., Ballinari P., Remonda L., Schroth G., Mattle H.P. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36(10):2121–2125. doi: 10.1161/01.STR.0000182099.04994.fc. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J., Zhao X., Wang A., Zhou Y., Yang B., Wei N., Yu D., Lu J., Chen S., Wang Y., Wang C., Xue R., Zhang Y., Li Y., Yu L., Wang S., Chen Z., Zheng T., Zhang Z., Xia M., He M., Li W., Zhang Z., Zeng F., Chen S., Fu Y., Liu G., Wang L., Huang Z., Ma J., Mu F., Xu Y., Huang R., Wang L., Wang Y. Emerging malnutrition during hospitalisation independently predicts poor 3-month outcomes after acute stroke: Data from a Chinese cohort. Asia Pac. J. Clin. Nutr. 2015;24(3):379–386. doi: 10.6133/apjcn.2015.24.3.13. [DOI] [PubMed] [Google Scholar]
- 19.Serra M.C. The importance of assessing nutritional status to ensure optimal recovery during the chronic phase of stroke. Stroke Res. Treat. 2018;2018:1297846. doi: 10.1155/2018/1297846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nozoe M., Kubo H., Kanai M., Yamamoto M. Relationships between pre-stroke sarc-f scores, disability, and risk of malnutrition and functional outcomes after stroke-a prospective cohort study. Nutrients. 2021;13(10):3586. doi: 10.3390/nu13103586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouziana S.D., Tziomalos K. Malnutrition in patients with acute stroke. J. Nutr. Metab. 2011;2011:167898. doi: 10.1155/2011/167898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siparsky P.N., Kirkendall D.T., Garrett W.E., Jr Muscle changes in aging: Understanding sarcopenia. Sports Health. 2014;6(1):36–40. doi: 10.1177/1941738113502296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candow D.G., Forbes S.C., Little J.P., Cornish S.M., Pinkoski C., Chilibeck P.D. Effect of nutritional interventions and resistance exercise on aging muscle mass and strength. Biogerontology. 2012;13(4):345–358. doi: 10.1007/s10522-012-9385-4. [DOI] [PubMed] [Google Scholar]
- 24.Abe T., Iwata K., Yoshimura Y., Shinoda T., Inagaki Y., Ohya S., Yamada K., Oyanagi K., Maekawa Y., Honda A., Kohara N., Tsubaki A. Low Muscle mass is associated with walking function in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2020;29(11):105259. doi: 10.1016/j.jstrokecerebrovasdis.2020.105259. [DOI] [PubMed] [Google Scholar]
- 25.Ohyama K., Watanabe M., Nosaki Y., Hara T., Iwai K., Mokuno K. Correlation between skeletal muscle mass deficit and poor functional outcome in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2020;29(4):104623. doi: 10.1016/j.jstrokecerebrovasdis.2019.104623. [DOI] [PubMed] [Google Scholar]
- 26.Shpata V., Ohri I., Nurka T., Prendushi X. The prevalence and consequences of malnutrition risk in elderly albanian intensive care unit patients. Clin. Interv. Aging. 2015;10:481–486. doi: 10.2147/CIA.S77042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abd-El-Gawad W.M., Abou-Hashem R.M., El Maraghy M.O., Amin G.E. The validity of geriatric nutrition risk index: Simple tool for prediction of nutritional-related complication of hospitalized elderly patients. comparison with mini nutritional assessment. Clin. Nutr. 2014;33(6):1108–1116. doi: 10.1016/j.clnu.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Dennis M. Nutrition after stroke. Br. Med. Bull. 2000;56(2):466–475. doi: 10.1258/0007142001903102. [DOI] [PubMed] [Google Scholar]
- 29.Mansare M.L., Kouda D.A., Diallo I.M., Bakhoum M., Mourabit S., Toure K., Ndiaye M., Diop A.G., Ndiaye M.M. What is the nutritional status of your patients suffering from strokes. Rev. Neurol. (Paris) 2020;176(5):366–369. doi: 10.1016/j.neurol.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Rabadi M.H., Coar P.L., Lukin M., Lesser M., Blass J.P. Intensive nutritional supplements can improve outcomes in stroke rehabilitation. Neurology. 2008;71(23):1856–1861. doi: 10.1212/01.wnl.0000327092.39422.3c. [DOI] [PubMed] [Google Scholar]
- 31.Mitrache C., Passweg J.R., Libura J., Petrikkos L., Seiler W.O., Gratwohl A., Stähelin H.B., Tichelli A. Anemia: An indicator for malnutrition in the elderly. Ann. Hematol. 2001;80(5):295–298. doi: 10.1007/s002770100287. [DOI] [PubMed] [Google Scholar]
- 32.Keller U. Nutritional laboratory markers in malnutrition. J. Clin. Med. 2019;8(6):E775. doi: 10.3390/jcm8060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabrerizo S., Cuadras D., Gomez-Busto F., Artaza-Artabe I., Marín- Ciancas F., Malafarina V. Serum albumin and health in older people: Review and meta analysis. Maturitas. 2015;81(1):17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Cereda E., Vanotti A. The new geriatric nutritional risk index is a good predictor of muscle dysfunction in institutionalized older patients. Clin. Nutr. 2007;26(1):78–83. doi: 10.1016/j.clnu.2006.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. [J.Z], upon author request.