Figure 7. Gpr17 regulates GLP-1 secretion through Ca2+ and cAMP signaling.
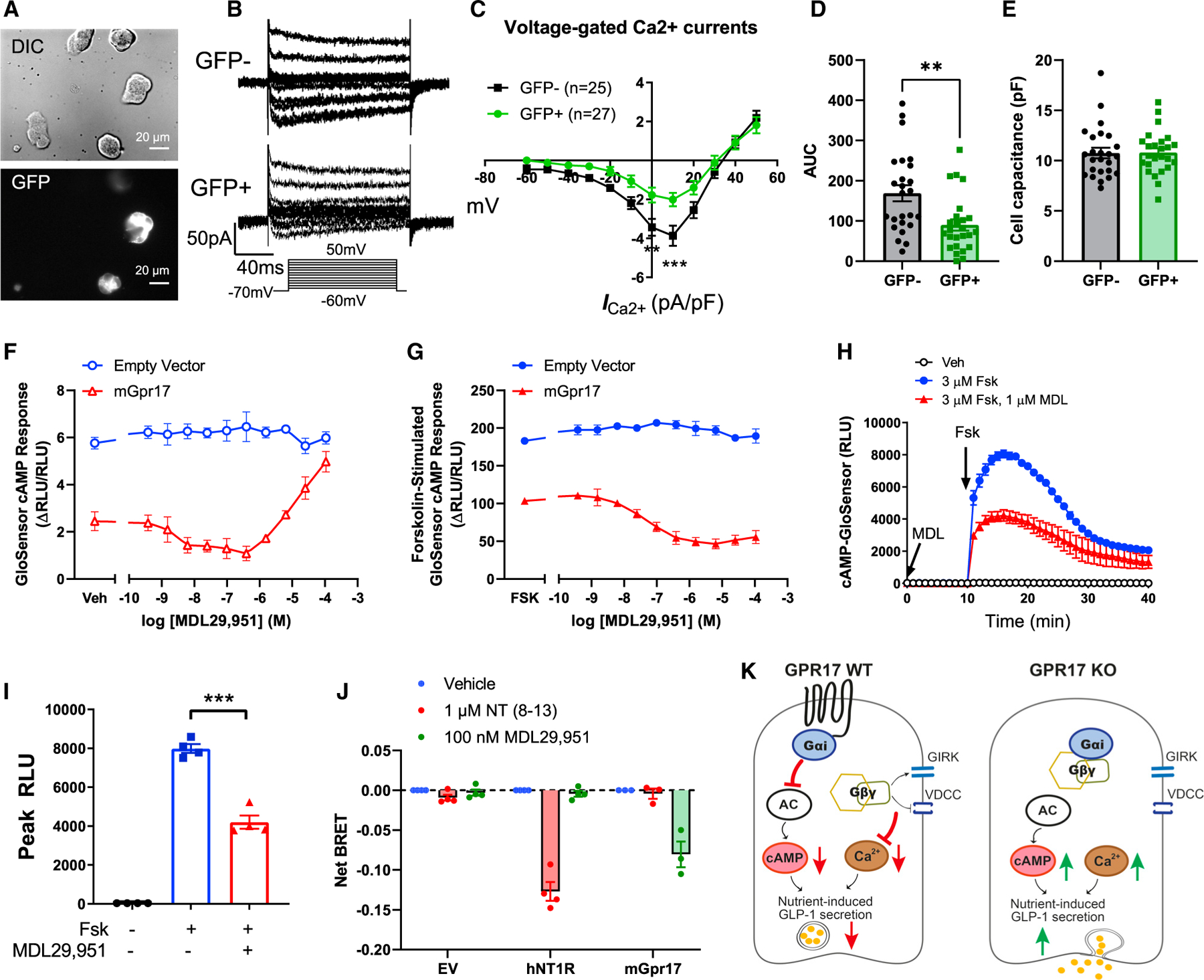
(A) Whole-cell patch clamp recording voltage-gated Ca2+ currents in GLUTag cells that were transiently transfected with pcDNA3.1-mGpr17-GFP. All cells were patched under IR-DIC optics and GFP-positive cells were identified by using an upright microscope fitted with fluorescence optics.
(B) Ca2+ current responses to 150 ms voltage steps of 10 mV increments from a holding potential of −70 mV. The inset shows the voltage pulse protocol. Tetrodotoxin (TTX) at 0.3 µmol/L was added to block Na+ currents.
(C) Current–voltage relationship of the voltage-gated Ca2+ currents recorded in Gpr17-overexpressed GLUTag cells (GFP+; n = 27 cells) and nontransfected cells (GFP−; n = 25 cells). Statistical comparisons between groups in were performed using two-way ANOVA and Sidak’s post hoc tests.
(D) Averaged AUC for voltage-current curve of Ca2+ currents.
(E) Cell capacitance were comparable between Gpr17-overexpressed GLUTag cells (GFP+) and nontransfected cells (GFP−).
(F) Gpr17 expression and agonist stimulation, MDL29,951, inhibited GloSensor cAMP response in GLUTag cells without forskolin addition.
(G) Gpr17 expression and MDL29,951 inhibited forskolin-stimulated GloSensor cAMP response in a concentration-dependent manner in GLUTag cells. Data for (F) and (G) were represented as the mean ± SEM of two independent experiments performed with duplicate wells.
(H and I) Time course recording showing MDL29,951 inhibited forskolin-stimulated cAMP response in mouse Gpr17-overexpressing GLUTag cells (n = 4 replicates) (H). Peak RLU were quantified (I). Vehicle (Veh) or MDL29,951 (1 µmol/L) were incubated for 10 min before forskolin addition. Unpaired two-tailed Student’s t test was performed.
(J) GPR17-Gαi/Gβγ coupling is identified by TRUPATH in HEK293 cells transiently transfected with either pcDNA3.1 (−) empty vector, pcDNA3.1 (+)-hNT1R, or pcDNA3-HA-mGpr17 together with pcDNA5/FRT/TO-Gαi1-Rluc8, pcDNA3.1-Gβ3, and pcDNA3.1-Gγ9-GFP2. The net BRET response represents the BRET ratio for each well subtracted by the mean BRET ratio response of the vehicle-treated wells for each receptor transfection condition. hNT1R, human neurotensin receptor type 1. Data are represented as the mean ± SEM of three or four independent experiments performed with triplicate wells.
(K) Schematic diagram illustrates the molecular mechanisms of how GPR17 negatively regulates nutrient-induced GLP-1 secretion in EECs. GPR17 signals through Gαi pathway to reduce cAMP production in enteroendocrine cells, in turn dampening nutrient-stimulated cAMP rise and Ca2+-stimulated exocytosis. Gβγ subunits released from Gαi reduce Ca2+ influx upon nutrient stimulation through activation of G-Protein-Coupled Inwardly Rectifying Potassium (GIRK) channels and inhibition of VDCCs. On the other hand, GPR17 deficiency increases cAMP and intracellular Ca2+ rise, thereby potentiating nutrient-induced GLP-1 secretion.
*p < 0.05, **p < 0.01, ***p < 0.001. Data are displayed as means ± SEM.
