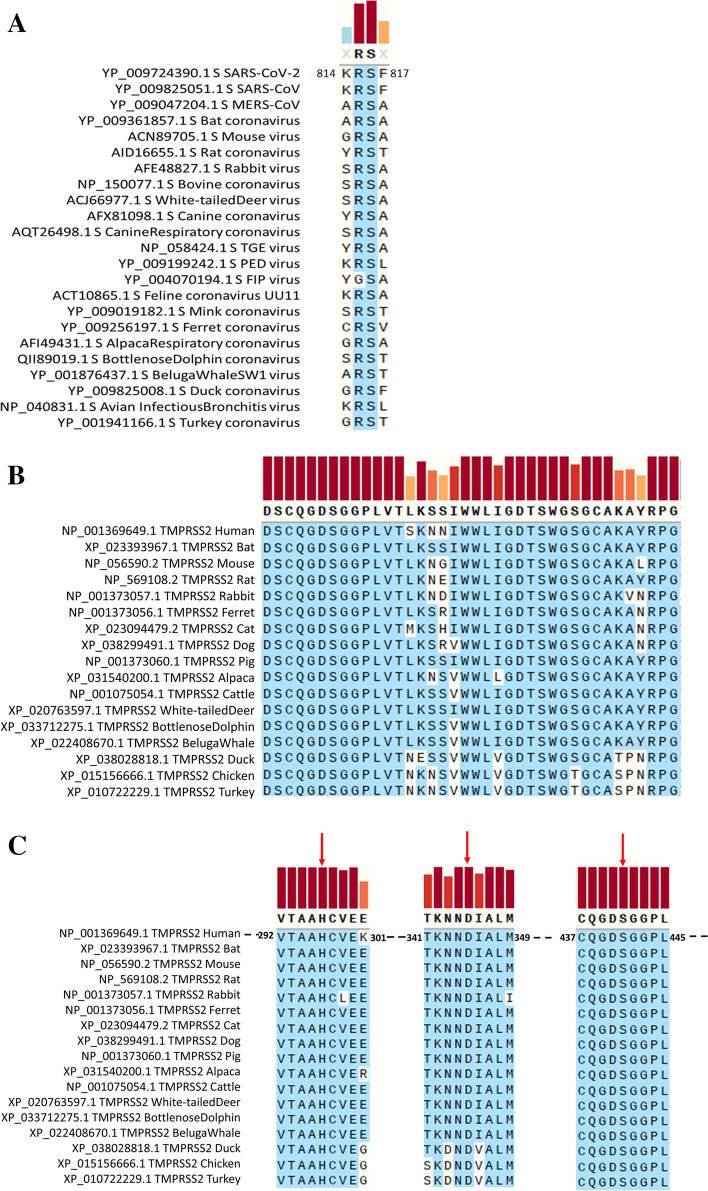
Fig. 3.
S protein cleavage site and catalytic residues of the TMPRSS2 enzyme are conserved. A S protein sequence alignment at the site where human TMPRSS2 cleaves the SARS-CoV-2 spike protein. The TMPRSS2 enzyme cleaves between the arginine (R) and serine (S) residues. Only the FIP virus has a glycine (G) in place of the arginine (R) residue. Aligned protein sequences of the substrate binding site (B) and active site (C) of the TMPRSS2 enzyme of various host species are shown. Arrows in panel C point to the triad of catalytic residues histidine (H) 296, aspartic acid (D) 345 and serine (S) 441 that are essential for binding to the SARS-CoV-2 S protein. All conserved residues are shaded blue, and sequence consensus conservation is shown as colored bars (red, tall bars mean more conserved). The threshold for showing a consensus is set at > 70 for A and > 50% for B and C. The letter X denotes no consensus, and amino acids are listed in single letter codes