Abstract
Menstrual health affects a large number of women throughout reproductive life since adolescence. Knowledge of the duration and variation of the menstrual cycle is necessary for patient education and to identify deviations from normal to guide clinical evaluation. The average duration of menstrual flow is between 4 to 6 days, with anormal range from 2 up to 8 days ; the mean blood loss per menstrual cycle is 25- 30 mL. In general, descriptive data falling outside the normal range are considered to be indicative of menstrual disorders. Although the American Academy of Pediatricshttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC5710410/ - poi170032r27 and American College of Obstetricians and Gynecologistshttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC5710410/ - poi170032r29 advocate for clinicians to consider the menstrual cycle as a vital sign in adolescents, the identification of subjects with hypomenorrhea is neither well defined nor routinely practiced. In this paper we have summarized the published prevalence of hypomenorrhea (lighter and/or shorter menstrual bleeding) in adolescents and youths in different countries and report the personal experience in four adolescents. (www.actabiomedica.it)
Keywords: Adolescents, menstrual blood loss, menstrual disorders, hypomenorrhea, menstrual health
Introduction
Adolescence presents a vulnerable period during which menstrual abnormalities may arise. The spectrum of menstrual and associated reproductive problems ranges from minor disorders to serious life-threatening diseases. These abnormalities may be related to the maturational processes of the neuroendocrine, gonadal, and anatomic components of the reproductive system (1-3). One study that reviewed menstrual records of 250 girls, (100 longitudinally for up to 6 yrs), showed that in the majority of adolescents menstrual regularity was established within 2 years after menarche (4). Other studies have suggested that 5–7 yrs of gynecological age are required to establish a reliable ovulatory pattern (5–7). Therefore, problem arises as to whether menstrual cycle irregularity is physiological in adolescence and when diagnostic procedures should be initiated to exclude pathology.
A careful history, physical examination and selected laboratory tests can help to differentiate transient menstrual irregularity from the large number of endocrine and anatomic abnormalities that are also present in this age group related to menstrual cycle.
Many of the published studies are restricted to the diagnosis and treatment of dysmenorrhoea, oligomenorrhoea associated with hyperandrogenism, premenstrual syndrome, hypothalamic amenorrhea, secondary amenorrhea, and abnormal uterine bleeding (AUB) (1-3,7,8). Although the American Academy of Pediatrics https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5710410/ - poi170032r27 and American College of Obstetricians and Gynecologists https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5710410/ - poi170032r29 advocates clinicians to consider the menstrual cycle as important information in adolescents (9), the identification of subjects with hypomenorrhea (lighter and/or shorter menstrual bleeding) is neither a well defined condition nor routinely look for in adolescent medicine.
Beginning with the physiology of the menstrual cycle, we have summarized the published prevalence of hypomenorrhea in adolescents and youths (10-24 years) in different countries and report personal experience in four adolescents. In addition, the potential long-term implications on reproductive health are also highlighted.
Menstrual cycle physiology: a summary of current concepts
Recent advances enriched considerably our knowledge of menstrual physiology. Understanding the regulation of menstruation is of clinical interest as regular menstrual cycles reflect normal functioning of the hypothalamic-pituitary-ovarian (HPO) axis.
Menstruation is the monthly shedding of the uterine endometrium lining that occurs along with the periodic hormonal changes of ovaries. The menstrual cycle is divided into three phases: follicular, ovulatory and luteal. Normal menstrual cycles require the following: (a) integrity of the hypothalamic-pituitary-gonadal axis, (b) normal ovaries, (c) endometrial response to ovarian steroids and (d) normal adrenal and thyroid function (10,11).
During the follicular phase, endometrial cells proliferate under the influence of estrogen, but following ovulation, progesterone secretion stimulates additional morphologic changes in the endometrium (12). Moreover, nonsteroidal substances, such as inhibin A and inhibin B, also have an effect.
Cumulative evidence has demonstrated that another nonsteroidal ovarian substance, gonadotrophin surge-attenuating factor (GnSAF), plays a key role in the control of LH secretion during the follicular phase and at midcycle, thus providing a novel aspect in the ovarian control of gonadotrophin secretion during the human menstrual cycle (13).
After an ovulatory cycle, menstruation is most often a result of progesterone withdrawal, which induces a series of events involving vasoconstriction, cytokine changes in the endometrium, and programmed cell death (11). Progesterone withdrawal during the late secretory phase of the menstrual cycle results in up-regulation of COX-2, an enzyme responsible for prostaglandin synthesis. Elevation of COX-2 induces synthesis of PGE2 and PGF2α. PGF2α is a potent vasoconstrictor and, along with other vasoconstrictors, such as endothelin-1, result in constriction of spiral arterioles. This causes a transient episode of hypoxia in the functional layer of the endometrium. The transcription factor hypoxia-inducible factor (HIF-1) plays critical roles in cellular responses to hypoxia, the generation of an inflammatory response and vasculogenesis through transcriptional activation of angiogenic genes. It is composed of two subunits: alpha (HIF-1α) and beta (HIF-1β). HIF-1α is regulated by oxygen and is rapidly degraded by the proteasome in normoxic conditions (14). The precise local mechanisms involved in this efficient repair have not yet been fully elucidated, although it has been proposed that hypoxia is involved in the initiation and optimization of postmenstrual repair factor expression after progesterone withdrawal (14).
Within two days after the start of menstruation and while endometrial shedding is still occurring, estrogen produced by the growing follicles starts to stimulate the regeneration of the surface endometrial epithelium. The estrogen secreted by the growing ovarian follicles causes prolonged vasoconstriction enabling the formation of a clot over the denuded endometrial vessels (15). The myometrium regulates the amount of blood during each menstruation because its contraction causes vasoconstriction and decreased blood supply to the endometrium, as well as the proliferation of the basal layer, which allows epithelialization of the endometrium (11,15). Within a period of 5-6 days the old lining is shed, and a new regenerated lining without scarring is produced; a remarkable example of controlled tissue remodeling not seen in other organs.
Menstrual fluid is composed of desquamated endometrial tissue, red blood cells, inflammatory exudates, and proteolytic enzymes.
During menstruation, the endometrial strip thickness measured by pelvic ultrasonography (US) appears as a thin, echogenic line of 1–4 mm. Once the proliferative phase of the menstrual cycle (days 6–14) begins, the endometrium becomes thicker (5–7 mm) (16). In the late proliferative (periovulatory) phase, the endometrium develops a multilayered appearance. In this stage, the endometrium may increase up to 11 mm. The layered appearance usually disappears 48 hours after ovulation. During the secretory phase, the endometrium becomes even thicker (7–16 mm) and more echogenic (17) (Figure 1).
Figure 1.
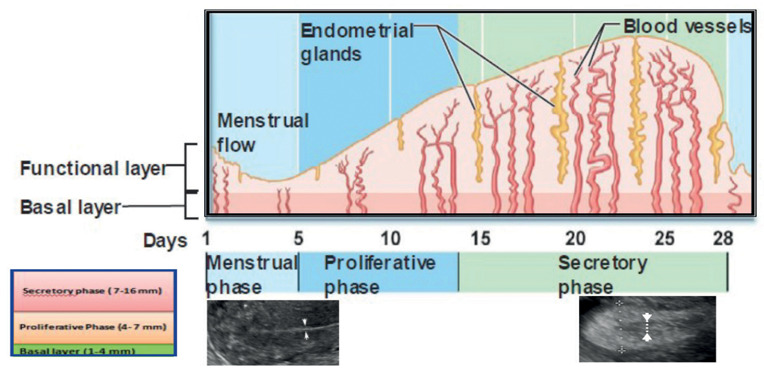
Pictorial essay of endometrium during menstrual cycle and endometrial thickness measurements (arrows) by pelvic ultrasonography during the early proliferative (left) and secretory phases (right) (Adapted from: De Sanctis V, Soliman A, Soliman R, Elalaily R, Millimaggi G. Riv Ital Med Adolesc 2015;13:30-3).
Characteristics of menstrual patterns throughout adolescence
The characteristics of the menstrual cycle are described in terms of frequency, regularity, duration, and amount of menstrual flow. The menstrual cycle is typically most irregular around the extremes of reproductive life (menarche and menopause) due to anovulation and inadequate follicular development.
Two large studies, one evaluating 275,947 cycles in 2,702 females and another 31,645 cycles in 656 females, support that menstrual cycles in adolescents, despite variability in the first gynecologic year, typically range from 21 to approximately 45 days (averaging 32 days) (18,19). However, by the third gynaecological year, 60–80% of girls have menstrual cycles 21– 35 days long as adult women (18,19).
The 5th percentile for cycle length, in the first gynecologic year, is 23 days and the 95th percentile 90 days. By the fourth gynecologic year, fewer females have cycles that exceed 45 days, but anovulation is still significant for some, with the 95th percentile for cycle length at 50 days. By the 7th gynecologic year, cycles are shorter and less variable, with the 5th percentile at 27 days and the 95th percentile at only 38 days. Thus, during the early years after menarche, cycles may be somewhat longer because of anovulation, but 90% of cycles will be within the range of 21 to 45 days (20,21).
The average duration of menstrual flow is between 4 to 6 days, but the normal range in women can vary from 2 days up to 8 days. The average amount of menstrual blood loss is 25- 30 mL per cycle. An amount greater than 80 mL is considered abnormal (22).
Knowledge of the length and variation of the menstrual cycle is necessary for patient education and for identifying deviations from normal to guide clinical evaluation. Moreover, evaluation of abnormal menstrual patterns throughout adolescence may permit early identification of potential health problems concerning adulthood. Therefore, clear definitions and terminology are important for clinical practice, and for epidemiologic studies of disorders of menstrual cycle (23).
A recent study was conducted to better assess cycle blood flow and duration in regularly menstruating women (24). The menstrual bleeding in total was classified in tertiles and defined as light: ≤ 36.5 mL, medium: > 36.5 and ≤ 72.5 mL, and heavy: >72.5 mL, and the daily individual bleeding as light: ≤ 4 mL, medium: > 4 and ≤ 14 mL, or heavy: > 14 mL.
Several methods have been suggested for assessment of menstrual blood loss (MBL), e.g.: counting the number of days of menstruation and the number of sanitary products, weighing sanitary products, and using the pictorial blood loss assessment chart (PBAC) (22, 25-27).
Despite evident limitations in accuracy, weighing used sanitary products and subtracting the weight of the unused product may be a simple method for the MBL estimation (28). In that case the menstrual fluid evaporation could be minimized by using airtight sealed bags (29).
The PBAC has an acceptable diagnostic accuracy compared to objective measurement of MBL. It is a semi-quantitative method for evaluation of MBL, mainly in subjects with abnormal uterine bleeding (30-32). The total amount of menstrual blood flow, expressed in mL, is estimated daily by calculating the sum of the scores of each sanitary napkin or tampon used using the scale shown in figure 2. The chart indicates not only the number but also the degree of soiled items of sanitary wear.
Figure 2.
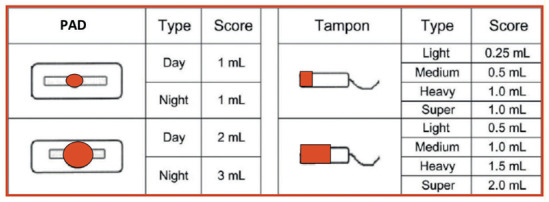
Assessment of menstrual blood loss by pictograms with blood loss equivalents. Adapted from: Wyatt KM, Dimmock PW, Walker TJ, et al. Determination of total menstrual blood loss. Fertil Steril. 2001;76:125–31.
However, the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) report stated that “a light menstrual bleeding is based on complaint by the patient “ (Table 1).
Table 1.
FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) classification of menstrual bleeding disorders (From: Ref. 25).
| Term | Classification | Definition |
|---|---|---|
| Disturbances of regularity |
Irregular menstrual bleeding
Absent menstrual bleeding |
> 20 days in individual cycle lengths over a period of 1 year. No bleeding in a 90-day period. |
| Disturbances of frequency |
Infrequent menstrual bleeding
Frequent menstrual bleeding |
One or two episodes in a 90-day period More than four episodes in a 90-day period. |
| Disturbances of heaviness of flow |
Heavy menstrual bleeding
Light menstrual bleeding |
Excessive menstrual blood loss which can occur alone or in combination with other symptoms. This is based on complaint by the patient. |
| Disturbances of the duration of flow |
Prolonged menstrual bleeding
Shortened menstrual bleeding |
Menstrual periods that exceed 8 days in duration on a regular basis. Menstrual bleeding of no longer than 2 days in duration. |
| Irregular non-menstrual bleeding | Irregular episodes of bleeding, often light and short, occurring between otherwise fairly normal menstrual periods. |
In conclusion, in adult women the definition of hypomenorrhea is based mainly on the duration of menstrual flow which lasts 2 days or less and on a woman’s self- assessment (24). However, the latter is too vague and cannot provide an accurate estimation of quantity of menstrual blood loss. Therefore, we believe that there is an urgent need for an international consensus for the diagnostic criteria of hypomenorrhea and for its validation in adolescents and youths.
Prevalence of lighter and/or shorter menstrual bleeding in adolescents and youths in different countries
A systematic literature review to ascertain the prevalence of lighter and/or shorter menstrual bleeding in adolescents and youths (bleeding duration ≤ 2 days and/or light entity of menstrual bleeding) in different countries was carried out for the period 1970 to September 2021. We searched general bibliographic databases: PubMed, Google Scholar and Embase. The following medical subject heading (MeSH) terms, keywords and their combinations for electronic databases were used: “light or short menstrual bleeding” or “hypomenorrhea” or “ menstrual disorders” were combined with terms like “definition” or “nomenclature” or “classification” or “prevalence” or “incidence” and “ adolescents” or “youths” or “young adults” and “different countries”. The full text of each selected article was reviewed. Any additional relevant study not identified by the electronic searches was considered.
Twenty-three studies including 41,827 adolescents and young adult women were eligible for review (Tables 2A-B and C,). All but two studies were from low, lower-middle or upper-middle-income countries. Different criteria were used to define hypomenorrhea: “duration of menstrual flow for 1-2 days or regularly timed bleeding but scanty in amount (Table 2-B: no.11 and 12); duration of menses <3 days with slight blood loss (using ≤ 1 pad/ day) (Table 2-B: no.16) or from 1-3 days duration of menstrual bleeding and from < 1 pad per day to ≤ 4 pads per day”. In eight studies, the duration of menstrual bleeding was not available or not reported.
Table 2-A.
Relevant data selected from different countries
| Study ID and Country | Study design | Number of subjects and age (yrs.) | Age (yrs.) at menarche | Duration of menstrual bleeding | Entity of menstrual bleeding |
|---|---|---|---|---|---|
| 1. Widholm O and Kantero RL. AOGS 1971;14:19-29. Scandinavia | Self- administered questionnaire. | 5,485 menstruating adolescent girls. Range: 10-20 yrs. |
N.A. | 2-3 days in the 1st gynecological year in 8.8 % and after the 5th gynecological year was 3.7 % . | N.A. |
|
2. FlugD et al. Ann Hum Biol 1984;6:495-508. Switzerland |
Prospective longitudinal study. | 140 girls (urban Swiss population). | 13.4 ± 1.1 yrs. | 2 or less in 1.6% (1st yr after menarche) and 0.7%, six yrs after menarche. | Mild in 16.3 % (1st yr after menarche) and 10.9 %, six yrs after menarche. |
|
3. Lee L K et al. Singapore Med J 2006; 47:869-74. Malaysia |
Self- administered questionnaire (7 districts). | 2,411girls. 15.4 ±1.8 yrs. (Range: 12- 19 yrs). |
12.3 ± 1.1 yrs. | 2 or less in 1.6%. | ≤4 pads per day in 70.8%. |
|
4. Zegeye DT et al. BMC Womens Health 2009;(9): 29. Northwest Ethiopia |
Self-administered questionnaire | 612 subjects. Range:14- 19 yrs. |
14.8 (13.9-15.3) yrs. | 4 ± 1.3 days wit a range of 2-7 days. 2-3 days in 262 subjects (46.7%). |
3 ± 1.8 (range 1-12) pads per day. |
|
5. Begum J et al. Dinajpur Med Col J 2009; 2: 37-43. Dinajpur, Bangladesh |
Anonymous questionnaire distributed by the researchers. | 174 students. 21.1 ± 1.6 yrs. Range:19-25 yrs. |
N.A. | 1 day in 0.57%. | N.A. |
|
6. Santos IS et al. BMC Womens Health. 2011;11: 26. Brasil |
Cross-sectional study. | 204 subjects Range: 18-24 yrs. |
Menarche between 11 and 12 yrs in 42.2%. | Average duration of menstrual period: 4.4 days (Range:1 -3 days). |
2.8 regular size sanitary pads per day in 17.6 %. |
|
7. Lee JC et al. Korean J Pediatr 2011;54:201-6. Seoul, Korea |
Self-administered questionnaire. | 538 students. 14- 18 yrs. Mean age:16.1 yrs. |
Mean age at menarche 12.6 yrs. | 1-2 days in 0.4%. | Low number of sanitary pads (≤3) in 24.3%. |
|
8. Karout et al. East Mediterr Health J 2012;18:346-52. Beirut, Lebanon |
Self-administered questionnaire. | 352 students. Mean age: 20.9 yrs. Range: 18 – 26 yrs). |
13.2 yrs. | < 3 days in 2%. | < 1 pad per day in 2.0 %. |
Legend : N.A. not available.
Table 2-B.
Relevant data selected from different countries
| Study ID and Country | Study design | Number of subjects and age (yrs.) | Age (yrs.) at menarche | Duration of menstrual bleeding | Entity of menstrual bleeding |
|---|---|---|---|---|---|
| 9.Dambhare DG et al. Glob J Health Sci 2012; 4:105-11. Maharashtra, Central India | Self-administered questionnaire | 561 adolescents. Rural and urban residence |
13.6 ± 0.8 yrs. | < 2 days in 1.6%. | N.A. |
|
10. Gumanga SK, Kwame-Aryee RA..Ghana Med J 2012;46:3-7. Accra, Ghana |
Self-administered questionnaire | 456 students. 16 ± 0.9 yrs. Range:14 -19 yrs. |
12.5 ±1.2 yrs. | 2 days in 0.9%. | N.A. |
| 12. Mohite RV and Mohite VR. Al Ameen J Med Sci 2013; 6 :213-18. Western Maharashtra, India. | Cross Sectional study. Face to face personal interview. | 107 college students of rural area (Karad). Range: 17-20 yrs. |
Mean age 14.1 yr. Range:12-17 yrs. |
1-2 days in 78.6% in girls with regular menstrual cycles and 21.3% in girls with irregular menstrual cycles. | Scanty in 73.7% with regular menstrual cycles and 26.2% in girls with irregular menstrual cycles. |
| 13. Sheetu MK et al. Sch J App Med Sci 2014; 2:529-34. Maharashtra, India | Cross sectional study. Subjects were then interviewed face to face. | 268 unmarried adolescents Range: 10-19 yrs. |
13.4 ± 1.0 yrs. | ≤ 2 was present in 10.5%, 1.9% and 4% of subjects with light, moderate and heavy episodes of bleeding. | N.A. |
| 14. Shiferaw MT et al. Pan African Medl J 2014; 17:246. Bahir Dar, Ethiopia | Self-administered questionnaire. | 470 subjects. Range: 17 - 24 yrs. |
N.A. | ≤ 2 days in 10.6%. | ≤4 pads per day in 84.6 %. |
| 15. Nirmala JL et al. Sch J App Med Sci 2014; 2:3165-75. Pondicherry, India | Self-administered questionnaire. | 200 female students. | 12.6 ± 1.3 yrs. | < 2 days in 8%. | ≤ 2 pads per day in 16%. |
|
16. Abdelmoty HI et al. BMC Womens Health 2015;15:70. Giza, Egypt |
Cross-sectional study. Self-administered questionnaire. | 412 students 14.6 ± 1.7 yrs. Range: 11–19 yrs. |
12.49 ± 1.20 yrs. | < 2 days in 1.9 %. | N.A. |
|
17. Nooh AM et al. Middle East Fertil Soc J 2015: 20: 198 –203. Zagazig, Egypt |
A random sample of female students questionnaires were distributed. | 283 students 17.8 ± 0.7 yrs. Range: 17–19 yrs. |
12.1 ± 1.6 yrs. Range: 11–16 yrs. |
< 3 days in 7.1%. | ≤ 1 pad/day in 9.1%. |
| Legend: N.A. not available. | |||||
Table 2-C.
Relevant data selected from different countries
| Study ID and Country | Study design | Number of subjects and age (yrs.) | Age (yrs.) at menarche | Duration of menstrual bleeding | Entity of menstrual bleeding |
|---|---|---|---|---|---|
| 18. Elnagar RR et al. Int J Nurs Didact 2017; 7: 27-37. Mansoura, Egypt | Structured interview questionnaire. | 986 nursing students. 20.1 ±1.2 yrs. Range:18 - 24 yrs. |
11 – 13 yrs in 68.3%. | < 2 days in 0.2%. | N.A. |
| 19. Zafar et al. IJPSR, 2018.9: 2088-99.Arifwala, Pakistan | Structured questionnaire. | 723 students. Range: 10 - 19 yrs. |
12 years in 35.4%. | <3 days in 13.3%. | 22.8%, light blood loss (using 1 pad/ daily). |
| 21. Duttal BK et al. J Evid Based Med Health 2018;5: 3239-44. Guwahati, India | Hospital based observational study. | 200 adolescents | 12.3 ± 1.0 yrs. | Hypomenorrhoea was prevalent in 3.5%. | Light menstrual flow in 3.5% (number of pads changed during menstrual flow). |
| 22.Shamloo S et al. Dis Diagn 2020;9:148-52. Bandar Abbas, Iran | Cross-sectional and descriptive-analytic study, a questionnaire was used to collect the data. | 370 students. 16.2 ± 0.89 yrs. Range: 15-18 yrs. |
12.8 ± 1.0 yrs. | < 2 days in 24.6%. | N.A. |
|
23. Mao L et al. Medicine 2021; 100:16. China |
Self-designed, semi-structured questionnaire. | 24,670 adolescents < 18 yrs. |
N.A. | ≤ 1 day in 3.9%, mainly in women aged <18 yrs. | Less than 5 pads per cycle was considered as hypomenorrhea: 8.1%. |
Legend : N.A. not available.
Hypomenorrhea was reported to be more common in underweight girls (Table 2-A: no. 2) or adolescents with regular menstrual cycles (Table 2-B: no.12). An extreme variability of hypomenorrhea was reported in different countries [e.g.: from 0.2 % in Egypt (Table 2-B: no. 17) to 24.6% in Iran (Table 2-C: no. 22)]. However, race, ethnicity, culture and lower social class may have influenced the expression of menstrual symptoms and the reported severity. The prevalence of short menstrual bleeding duration and/or light menstrual bleeding improved after the 5th- 6th gynecological year (Table 2-A: no.1 and 2).
The principal limitations reported in the selected studies were the variability of diagnostic criteria for the definition of hypomenorrhea, the study characteristics for data collection (questionnaires were employed suggesting an important risk of information bias in reporting the characteristics of vaginal bleeding) and volunteerism that could differ from the study populations. Moreover, none of the studies reported the potential impact of environmental toxins or air pollution exposure on the menstrual cycle characteristics.
Nevertheless, data in table 2 add relevant information to a neglected aspect of menstrual history of adolescents and youths, and may provide a window of opportunity for early diagnosis and treatment of disorders that could have long-term adverse health consequences.
Personal experience in four patients
Patient 1:
A 17-year- old female complained of of “short periods” (1-2 days) since menarche (at age 12 years). In the past, she had believed that her menstrual flow had been “more or less” regular because of her “young menstrual age”.
Personal history was unremarkable. The menstrual intervals were 28 ± 5 days. There was no history of emotional stress, digestive disorders, sharp weight loss or intense physical activity. Dysmenorrhea was present in the first two days of menstrual cycle and acetaminophen was occasionally taken. Her mother experienced menarche at the age of 13 years. There was no family history of menstrual flow disorders, gynecological tumors or other gynecological problems.
On examination, she was well developed (height and weight: 25th and 35th percentile for age respectively). Blood pressure was 110/50 mmHg. She did not have a palpable goitre, acne or hirsutism. Breasts were at Tanner’s stage 4 and pubic hair at Tanner’s stage 5 of development . External genitalia were normal with normal tenderness on pelvic examination.
The pictorial blood loss assessment chart used for 3 months confirmed normal menstrual interval and the presence of short duration of menstrual flow. The basal body temperature (BBT) taken orally with a digital thermometer at the same time, every morning for 2 menstrual cycles, showed a normal biphasic curve.
Abdominal and pelvic ultrasound revealed normal uterus and ovaries. Endometrial thickness was measured at the widest point in the longitudinal plane, including all of the highly reflective endometrium. The thickness in the proliferative phase of the menstrual cycle (6–14 days) was 5 mm and in the late proliferative (periovulatory) phase was 7 mm.
Routine biochemical laboratory blood tests were normal. Endocrine laboratory tests, performed in the early follicular phase of the menstrual cycle, showed: total testosterone: 40 ng/ dL (nv: 7-50 ng/dL), androstendione 1.6 ng/mL (nv: 0.4-2.7 ng/mL), dehydroepiandrosterone -sulfate (DHEA-S): 155 μg/dL (n.v.: 16-304 μg/ dL), 17-hydroxyprogesterone: 0.6 ng/mL (n.v.: 0.1-1.5 ng/mL), prolactin: 12 ng/dL (n.v.: 0-25 ng/mL), LH: 2.9 mUI/mL (n.v.: 1.7-14mUI/mL), FSH: 3.3 mUI/ mL (n.v.: 3.9-10 mUI/mL), estradiol: 46 pg/mL (range from 20 to 80 pg/mL during the early to mid-follicular phase of the menstrual cycle), TSH: 3.1 μU/mL (n.v.: 0.25- 4 μU/mL), cortisol 18 μg/dL (n.v.: 7-20 μg/dL).
Transglutaminase antibodies (TTG), fecal calprotectin and pregnancy tests were negative.
Follow-up
No significant variations of menstrual interval, duration of flow and entity of menstrual bleeding were observed during 6 years of follow-up.
Patient 2:
A 15.5 year- old girl was referred for a second opinion because of short duration of menstrual flow. She said that she had had “light periods” (2-3 small pads/day for 1 and half - 2 days), since menarche that occurred at the age of 11.5 years.
Her personal history was unremarkable. The menstrual intervals were normal (from 31 to 33 days). There was no history of emotional stress, digestive disorders, sharp weight loss, intense physical activity or drug intake. She was not sexually active.
Her mother experienced menarche at the age of 11.2 years. There was no family history of menstrual flow disorders, gynecological tumors or other gynecological problems. Her grandmother had obesity and diabetes mellitus type 2.
On physical examination, her height was at 5th percentile, weight 15th percentile. Her blood pressure was 100/55 mm/Hg. She did not have acne or hirsutism. Breasts and pubic hair were at Tanner’s stage 5. Genitalia examination revealed normal outward appearance. The rest of her examination was normal.
Routine biochemical laboratory blood tests were in the normal range. Endocrine laboratory data, assayed in the early follicular phase of the menstrual cycle, were as follows: total testosterone: 30 ng/ dL (n.v.: 7-50 ng/dL), androstendione 1.3 ng/mL (nv: 0.4-2.7 ng/mL), dehydroepiandrosterone-sulfate (DHEA-S): 160 μg/dL (n.v.: 16- 304 μg/ dL), 17-hydroxyprogesterone: 0.5 ng/mL (n.v.: 0.1-1.5 ng/mL), prolactin: 14 ng/dL (n.v.: 4-25 ng/mL), LH: 2.1 mUI/mL (n.v.: 1.7-14 mUI/mL), FSH: 5.3 mUI/ mL (n.v.: 3.9-10 mUI/mL), estradiol: 41 pg/mL (range from 20 to 80 pg/mL during the early to mid-follicular phase of the menstrual cycle), TSH: 1.51 μU/mL (n.v.: 0.25-4 μU/mL), cortisol 13 μg/dL (n.v.: 7-20 μg/dL). A pelvic ultrasound was done one year later. In the late proliferative (periovulatory) phase the endometrium thickness was 5 mm. Transglutaminase (TTG) antibodies and fecal calprotectin resulted negative
Follow-up
No significant variations of menstrual interval, duration of flow and entity of menstrual bleeding were observed during 5.5 years of follow-up.
Epicrisis
A diagnosis of “hypomenorrhea of unidentified etiology” was made in both patients. No significant variation of menstrual interval, duration of flow and entity of menstrual bleeding were registered during the follow-up.
Patient 3:
A 16-year-old girl with β-thalassemia major was referred for a second opinion because of hypomenorrhea (“lighter menstrual bleeding and two days duration of menstrual cycles”, occurring every 50-60 days). She had menarche at the age of 13 years and in the first two years of menstrual history the menstrual cycle length varied from 38 to 45 days. She did not complain of dysmenorrhea and was not sexually active. She was regularly transfused every 2-3 weeks to maintain a mean pre-transfusional hemoglobin (Hb) level around 9 g/dL. Daily subcutaneous chelation therapy with desferrioxamine, started at the age of 2 years, was replaced at the age of 10 years by deferiprone (75 mg/Kg body weight given orally).
On physical examination, her height was at 3rd percentile and body mass index was 18.5 kg/m2 (mild thinness). The blood pressure was 100/55 mm/Hg. Neither acne nor hirsutism was present. Breasts and pubic hair were at Tanner’s stage 4 and 3, respectively.
Her basal gonadotropins, 20 days after the last spontaneous menstrual cycle, were low (LH: 1.7 mUI/mL; n.v.: 1.7-14 mUI/mL and FSH: 2.3 mUI/ mL; n.v.: 3.9-10 mUI/mL). Plasma 17 β- estradiol was 23 pg/ml (normal range from 20 to 80 pg/mL during the early to midfollicular phase of the menstrual cycle). Basal hormonal evaluation revealed a normal level of prolactin (14 ng/dL: n.v.: 4 - 25 ng/mL), TSH (3. 4 μU/mL; n.v.: 0.25-4 μU/mL) and cortisol 11 μg/dL (n.v.: 7-20 μg/dL).
Her last serum ferritin level was 1,779 ng/mL and the peak registered in the peripubertal age was 2,990 ng/mL. The 90 th percentile of reported normal values in females is 201 ng/mL (33). Liver iron concentration LIC was 7 mg/g/dry weight (d.w.). Four classes of LIC have been reported in thalassemic patients: Class 1 = normal - LIC < 3 mg Fe/g/d.w., Class 2 = mild overload- LIC 3–7 mg Fe/g d.w., Class 3 = moderate overload- LIC 7–15 mg Fe/g d.w., and Class 4 = severe overload- LIC ≥15 mg Fe/g d.w. (34).https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6223579/ - b5-mjhid-10-1-e2018062
Serum concentrations of alanine aminotransferase (ALT) was mildly increased (56 U/L) and anti-HCV antibodies were negative. Fasting plasma glucose was 92 mg/dL. Magnetic resonance imaging of the pituitary gland did not show any significant abnormality, but a reduction of signal intensity on T2*-weighted images, as a marker of pituitary iron overload, was present.
Follow-up
After 18 months with no significant variation of oligo-hypomenorrhea she developed secondary amenorrhea characterized by poor response of LH and FSH to Gn-RH stimulation test and low level
of 17 β- estradiol associated with a negative medroxyprogesterone acetate test (MAP test: 10 mg given orally for 7 days), in the presence of a normal PRL and TSH. Her bone mineral density was reduced, showing osteopenia. A diagnosis of acquired central hypogonadism was diagnosed, mainly linked to chronic exposure to iron overload. A 17 β- estradiol transdermal patch associated with oral medroxyprogesterone acetate for 12–14 days every 30 days was prescribed.
It has been reported that iron deposition in the pituitary gland starts in the first decade of life and has a cytotoxic effect, leading to hypogonadotropic hypogonadism (34,35). Low body mass percentile and reduced intake of macro and micronutrients could be potential additional factors responsible for menstrual cycle irregularities in β-thalassemia major patients (36). The nutritional history of our patient did not document relevant deficiencies; however, we cannot fully exclude a reduced intake of some trace elements.
Patient 4:
A 13.5 year-old girl presented with “light monthly periods” from the age of menarche (13.2 years). She was not sexually active and had occasional hot flushes in the last 2 months. Moreover, there was no history of significant illness or surgery, and no family history of premature menopause. On clinical examination she was fully sexually mature and no abnormal findings were present. Her height and weight were at 25th percentile.
Investigations revealed that follicle-stimulating hormone was 88 U/L (FSH: menopausal range 20-140 U/L); luteinizing hormone was 35 U/L (LH: menopausal range 10-65 U/L); 17 β- estradiol
was low (12 pg /mL; range from 20 to 80 pg/mL during the early to mid-follicular phase of the menstrual cycle).
Full blood count, renal, liver and thyroid function, total testosterone, morning cortisol and prolactin were normal. Adrenal antibodies, thyroid peroxidase and thyroglobulin antibodies were negative.
Pelvic ultrasound was unremarkable apart from the absence of visible ovarian follicles. Karyotype was 46 XX and Fragile X (Cytosine-Guanine-Guanine) testing was normal. Anti-Mullerian hormone, antiovarian antibodies and galactosaemia testing were not assessed. Bone mineral density scan was reported normal for age, height and weight.
Follow-up
Menstrual bleeding ceased at the age of 14 years in the confirmed presence of hypergonadotropinemia. Premature ovarian insufficiency (POI) was diagnosed at 14.2 years of age. POI affects 1 in 10,000 adolescents and young women under the age of 20 years, The etiology of POI may be of genetic, iatrogenic, or autoimmune origin; however, in 60-70% of cases the cause is idiopathic (37). In adolescents, it is mainly iatrogenic, being linked to chemotherapy and/or radiotherapy for malignant neoplasms, or due to some chromosomal alteration, as in Turner syndrome (TS) or fragile X mutation (38,39).
Transdermal hormone replacement therapy was prescribed and implications for future childbearing and the need for periodic review were discussed. She was also advised on bone strength preservation (adequate calcium intake, vitamin D, exercise, avoiding smoking, alcohol consumption, and low body weight).
In conclusion, understanding what constitutes a normal menstrual cycle during the first gynecological years is a common problem for clinicians. The workup of an adolescent with menstrual dysfunction is directed toward separating the “functional” irregular menstrual pattern of an immature HPO axis from the large number of endocrine and anatomic abnormalities that can become manifest in this age group (Table 3).
Table 3.
Causes of lighter and/or shorter menstrual bleeding
|
Evaluation of an adolescent female suspected of having a menstrual disorder requires a careful medical history analysis that includes: age of onset and progression of pubertal development, age at menarche, history of menstrual cycle irregularity and the onset of sexual activity associated to physical examination, and in selected cases (suspected origin of the dysfunction, i.e.: outflow tract, ovary, or hypothalamus/pituitary) and laboratory and diagnostic tests. A normal menstrual cycle should be defined according to the following parameters: (a) regularity of menses, (b) frequency of menses, (c) heaviness of menstrual flow, and (d) duration of menstrual flow. In order to precisely determine the magnitude of the changes, the menstrual pattern should be documented, preferably by recording of menstruation in a schedule describing the interval, duration and amount of flow of at least the last three menstrual cycles. The information on outcomes of adolescent menstrual disorders is important as a guide to providing appropriate investigation, treatment, and follow-up.
What are the potential implications of hypomenorrhea for fertility?
Few studies have considered whether menstrual cycle characteristics are associated with fertility or pregnancy outcome. Although there are few information, it has been postulated that low follicular-phase estrogen is associated with lower fertility. Additionally, short bleeds may indicate an insufficient buildup of the endometrium that may not be adequate for implantation, resulting in reduced fecundity (40).
Small et al. (40) studied prospectively 470 women to determine whether cycle length or bleed length were associated with fertility or spontaneous abortion. Cycles with 5 days of menstrual bleeding had the highest fecundity and cycles up to 4 days of bleeding had lower fecundity.
A larger prospective study investigated the relationship between age at menarche, menstrual cycle length, menstrual bleeding duration and time to pregnancy in a cohort of 391,320 rural Chinese women. Later onset of menarche, longer menstrual cycle length, both shorter (<4 days) and longer (>5 days) bleeding duration were associated with a lower fecundability ratios (FR) and longer time to pregnancy (41).
To investigate endometrial receptivity (ER), conception outcome, and other factors among women with light menstrual bleeding of unidentified etiology, Gao et al. (42) studied 2,014, women (aged 20-35 years, without any history of pregnancy or desire to conceive, follicle-stimulating hormone <10 IU on second or third day of cycle, and regular menstrual periods). Participants with a menstrual blood volume of 5-20 mL formed the study group while the others the control group. Participants were followed up for 1 year to establish conception outcome. Women with lighter menstrual bleeding of unidentified etiology had an increased prevalence of low ER and poor conception outcome.
On the contrary, in the study of Wise et al. (43) performed on 2,653 Danish women enrolled from 2007 to 2009, the age at menarche, time to menstrual regularization, and duration or intensity of menstrual flow were not appreciably associated with fecundity. Similar results were reported by Wesselink et al. (44) in 2,189 female pregnancy planners, aged 21-45 years, in United States and Canada.
Conclusion
The menstrual cycle is a vital sign whose normality suggests overall good health while abnormality provides a window for early diagnosis and treatment of disorders that may have long-term adverse health consequences. Nevertheless, young patients, their parents and many clinicians are often unsure about what represents normal menstrual patterns, including what constitutes normal ranges for menstrual cycle length and amount and duration of flow through adolescence. Menstrual disturbances in adolescents may be multifactorial and should be carefully taken in consideration because not always are simply signs of HPO immaturity (45).
Reviewing the literature we noticed a variable or inconsistent definition of hypomenorrhea and very little information on its outcome.
Adolescents frequently do not keep accurate records of menstrual periods; therefore asking them to chart their menses may be beneficial, especially if the bleeding history is too vague or is considered inaccurate. However, we should keep in mind that using self-reported data (counting sanitary products or objective indicator for blood loss i.e. number of pads/tampons per menses) preclude an accurate assessment of light bleeding data due to variability in size and type of pads/tampons, hygiene patterns between women, frequency of attention to the entity of menstrual flow and income status.
In the last decade(s), duration and amount of menstrual bleeding has been used as fertility indicators, reflecting growth of the endometrial lining and the occurrence of ovulation (24). Shorter bleeds may indicate unsuitable endometrium for implantation, and longer bleeds anovulatory cycles (46-48). Although shorter (<4 days) and longer (>5 days) bleeding duration were associated with a lower FR in adulthood is associated with anovulation and infertility (39,40), it is not known whether a history of lighter and/or shorter menstrual bleeding in adolescents and youths, in early menstrual life, may affect fertility potential across the reproductive years.
In conclusion, a number of questions still remain open and many others have not yet fully answered. Hence it is imperative to promote longitudinal studies in subjects with hypomenorrhea to assess their potential health concerns during reproductive age to fill the many gaps in scientific knowledges about the characteristics of menstrual cycle.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Greydanus DE, McAnarney ER. Menstruation and its disorders in adolescence. Curr Probl Pediatr. 1982;12:1–61. doi: 10.1016/0045-9380(82)90034-2. [DOI] [PubMed] [Google Scholar]
- Kustin J, Rebar RW. Menstrual disorders in the adolescent age group. Prim Care. 1987;14:139–66. [PubMed] [Google Scholar]
- Engle E, Shelesnyak M. First menstruation and subsequent menstrual cycles of pubertal girls. Hum Biol. 1934;6:431–53. [Google Scholar]
- Zhang K, Pollack S, Ghods A, et al. Onset of ovulation after menarche in girls: a longitudinal study. J Clin Endocrinol Metab. 2008;93:1186–94. doi: 10.1210/jc.2007-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126. [PubMed] [Google Scholar]
- Vollman R. The menstrual cycle. Major Probl Obstet Gynecol. 1977;7:1–193. [PubMed] [Google Scholar]
- Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol. 1983;97:213–19. doi: 10.1677/joe.0.0970213. [DOI] [PubMed] [Google Scholar]
- Foster C, Al-Zubeidi H. Menstrual Irregularities. Pediatr Ann. 2018;47:e23–e8. doi: 10.3928/19382359-20171219-01. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Adolescence; American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–50. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. 2018 Aug 5. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc; 2000. [Google Scholar]
- Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. Physiology of the Endometrium and Regulation of Menstruation. Physiol Rev. 2020;100:1149–79. doi: 10.1152/physrev.00031.2019. [DOI] [PubMed] [Google Scholar]
- Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online. 2014;28:714–22. doi: 10.1016/j.rbmo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Hirani N, Jabbour HN, Critchley HO. Novel roles for hypoxia and prostaglandin E2 in the regulation of IL-8 during endometrial repair. Am J Pathol. 2011;178:1245–56. doi: 10.1016/j.ajpath.2010.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser IS. Regulating menstrual bleeding. A prime function of progesterone. J Reprod Med. 1999;(44 (2 Suppl)):158–64. [PubMed] [Google Scholar]
- Fleischer AC. Sonographic assessment of endometrial disorders. Semin Ultrasound CT MR. 1999;20:259–66. doi: 10.1016/s0887-2171(99)90071-9. [DOI] [PubMed] [Google Scholar]
- Hall DA, Yoder IC. Ultrasound evaluation of the uterus. In: Callen PW, editor. Ultrasonography in obstetrics and gynecology. 3rd ed. Philadelphia, Pa: Saunders; 1994. pp. 586–614. [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126. [PubMed] [Google Scholar]
- Vollman RF. The menstrual cycle. Major Probl Obstet Gynecol. 1977;7:1–193. [PubMed] [Google Scholar]
- World Health Organization Task Force on Adolescent Reproductive Health. World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. Longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. J Adolesc Health Care. 1986;7:236–44. [PubMed] [Google Scholar]
- Widhom O, Kantero RL. A statistical analysis of the menstrual patterns of 8,000 Finnish girls and their mothers. Acta Obstet Gynecol Scand. 1971;(14 (Suppl. 14)):1–36. [PubMed] [Google Scholar]
- Hallberg L, Högdahl AM, Nilsson L, Rybo G. Menstrual blood loss--a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966;45:320–51. doi: 10.3109/00016346609158455. [DOI] [PubMed] [Google Scholar]
- Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29:383–90. doi: 10.1055/s-0031-1287662. [DOI] [PubMed] [Google Scholar]
- Dasharathy SS, Mumford SL, Pollack AZ, et al. Menstrual bleeding patterns among regularly menstruating women. Am J Epidemiol. 2012;175:536–45. doi: 10.1093/aje/kwr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher U, Schumacher J, Mellinger U, Gerlinger C, Wienke A, Endrikat J. Estimation of menstrual blood loss volume based on menstrual diary and laboratory data. BMC Womens Health. 2012;12:24. doi: 10.1186/1472-6874-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt KM, Dimmock PW, Walker TJ, O’Brien PM. Determination of total menstrual blood loss. Fertil Steril. 2001;76:125–31. doi: 10.1016/s0015-0282(01)01847-7. [DOI] [PubMed] [Google Scholar]
- Hallberg L, Nilsson L. Determination of Menstrual Blood Loss. Scand J Clin Lab Invest. 1964;16:244–8. [PubMed] [Google Scholar]
- Pendergrass PB, Scott JN, Ream LJ. A rapid, noninvasive method for evaluation of total menstrual loss. Gynecol Obstet Invest. 1984;17:174–8. doi: 10.1159/000299144. [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir BR, Hjaltalin EF, Bragadottir G, Hauksson A, Geirsson RT, Onundarson PT. Quantification of menstrual flow by weighing protective pads in women with normal, decreased or increased menstruation. Acta Obstet Gynecol Scand. 2009;88:275–9. doi: 10.1080/00016340802673162. [DOI] [PubMed] [Google Scholar]
- Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–9. doi: 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- Janssen CA, Scholten PC, Heintz AP. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol. 1995;85:977–82. doi: 10.1016/0029-7844(95)00062-V. [DOI] [PubMed] [Google Scholar]
- Janssen CAH. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Eur J Obstet Gynecol Reprod Biol. 1996;70:21–22. doi: 10.1016/s0301-2115(96)02570-5. [DOI] [PubMed] [Google Scholar]
- Fulwood R, Johnson CL, Bryner JD. Hematological and nutritional biochemistry reference data for persons 6 months-74 years of age: United States, 1976-80. Vital Health Stat. 1982;11:1–173. [PubMed] [Google Scholar]
- Verlhac S, Morel M, Bernaudin F, Béchet S, Jung C, Vasile M. Liver iron overload assessment by MRI R2* relaxometry in highly transfused pediatric patients: an agreement and reproducibility study. Diagn Interv Imaging. 2015;96:259–64. doi: 10.1016/j.diii.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Hekmatnia A, Radmard AR, Rahmani AA, Adibi A, Khademi H. Magnetic resonance imaging signal reduction may precede volume loss in the pituitary gland of transfusion-dependent beta-thalassemic patients. Acta Radiol. 2010;51:71–7. doi: 10.3109/02841850903292743. [DOI] [PubMed] [Google Scholar]
- Di Maio S, Marzuillo P, Mariannis D, et al. A Retrospective Long-Term Study on Age at Menarche and Menstrual Characteristics in 85 Young Women with Transfusion-Dependent β-Thalassemia (TDT) Mediterr J Hematol Infect Dis. 2021;13(1):e2021040. doi: 10.4084/MJHID.2021.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff A, Christin-Maitre S. Insuficiencia ovárica prematura. EMC - Ginecología-Obstetricia. 2019;55:1–10. [Google Scholar]
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124:193–7. doi: 10.1097/01.AOG.0000451757.51964.98. [DOI] [PubMed] [Google Scholar]
- Pederson J, Kumar RB, Adams Hillard PJ, Bachrach LK. Primary ovarian insufficiency in adolescents: a case series. Int J Pediatr Endocrinol. 2015;2015:13. doi: 10.1186/s13633-015-0009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CM, Manatunga AK, Klein M, Feigelson HS, Dominguez CE, McChesney R, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology. 2006;17:52–60. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang YY, Zhang Y, et al. The influence of age at menarche, menstrual cycle length and bleeding duration on time to pregnancy: a large prospective cohort study among rural Chinese women. BJOG. 2017;124:1654–62. doi: 10.1111/1471-0528.14469. [DOI] [PubMed] [Google Scholar]
- Gao Y, Hong X, Wang Z, Zhu Y. Endometrial receptivity and conception outcome among women with light menstrual bleeding of unidentified etiology. Int J Gynaecol Obstet. 2018;140:37–41. doi: 10.1002/ijgo.12335. [DOI] [PubMed] [Google Scholar]
- Wise LA, Mikkelsen EM, Rothman KJ, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. 2011;174:701–9. doi: 10.1093/aje/kwr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselink AK, Wise LA, Hatch EE, et al. Menstrual cycle characteristics and fecundability in a North American preconception cohort. Ann Epidemiol. 2016;26:482–7. doi: 10.1016/j.annepidem.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popata VB, Prodanova T, Calisb KA, Nelson LM. The Menstrual Cycle A Biological Marker of General Health in Adolescents. Ann N Y Acad Sci. 2008;1135:43–51. doi: 10.1196/annals.1429.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiol Rev. 1995;17:265–86. doi: 10.1093/oxfordjournals.epirev.a036193. [DOI] [PubMed] [Google Scholar]
- Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13:668–74. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Scheike T, Keiding N, et al. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10:422–8. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]


