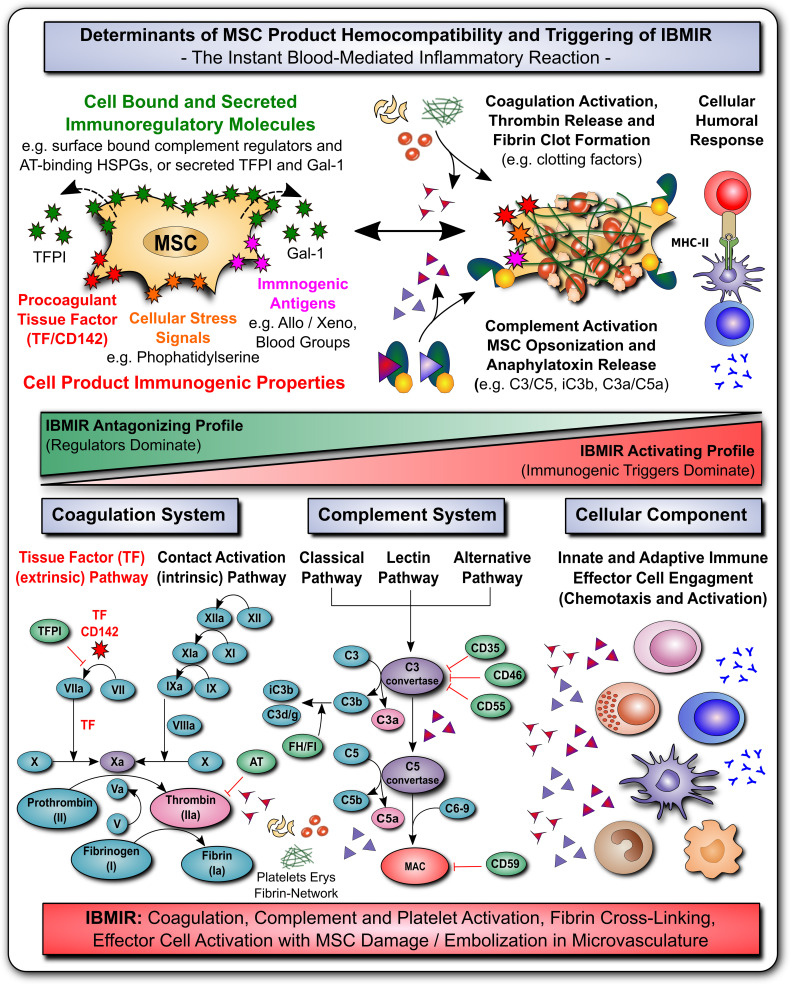
Figure 3.
MSC Determinants of Immunogenicity, Hemocompatibility and Interaction with the Innate Immune Cascade Systems. (A) The safety and efficacy of infused mesenchymal stromal cell (MSC) products depends on their hemocompatibility profile and concomitant triggering of the instant blood-mediated inflammatory reaction (IBMIR). In analogy to their immunomodulatory features, the cells display a broad array of either regulatory elements or immunogenic triggering factors (e.g. cell bound or secreted regulators of complement and coagulation cascade and cellular immunity). A tight balance between triggering and regulatory elements is decisive for the triggering of IBMIR (incompatibility with blood) or prevention thereof (hemocompatibility). While multiple blood regulatory elements employed by MSCs prevent blood activation akin to mechanism employed by hemocompatible endothelial cells, therapeutic MSC products can also display varying levels of immunogenic triggers, such as highly procoagulant tissue factor (TF/CD142), cellular stress signals (e.g. cell surface exposure of complement and coagulation activating phosphatidylserine resulting from membrane asymmetry upon freeze-thawing), and immunogenic antigens (e.g. allo-, xeno-, and blood group antigens). If these triggers prevail over the regulatory elements, blood-incompatible MSC products that are introduced into the blood stream can trigger the IBMIR, entailing the activation of complement, coagulation, and cellular immune responses, which may compromise MSC product safety and functionality. (B) Considering the coagulation cascade, regulatory elements entail amongst others the secreted tissue factor pathway inhibitor (TFPI) and surface localized heparan-sulfate proteoglycans (HSPGs) that bind antithrombin (AT), which are both strong negative-regulators of the highly procoagulant thrombin (activator of fibrin and platelets). Thrombin is formed upon triggering of the clotting cascade by activation of the extrinsic tissue factor pathway of coagulation (initiated by conversion of factor FVII to FVIIa), or the intrinsic contact activation pathway of coagulation (initiated through conversion of FXII to FXIIa, e.g. upon blood exposure of highly negatively charged basement membrane contained collagen residues). Both arms of the coagulation cascade converge where FX is turned into FXa, that promotes the conversion of prothrombin to thrombin, which in turn elicits conversion of fibrinogen to fibrin, that will form the fibrin clot through cross-linking fibrin fibers, and incorporating activated platelets, erythrocytes, and nucleated white blood cells. (C) Considering the complement cascade, regulatory elements entail the cell surface bound complement regulators CD35, CD46, CD55, CD59 and the secreted complement regulators factor H and I (FH/FI), that can regulate the cascade at different steps, as indicated in the figure, and may thus prevent formation of the final membrane attack complex (MAC) albeit initiation of the earlier steps of complement cascade activation. Activation of the complement cascade can occur via the classical, lectin, and alternative pathways of coagulation, triggered among others via recognition of aberrant cell surface features (e.g. phosphatidylserine exposure upon freeze-thawing) or bound immunoglobulins via C1q (classical pathway), which is then transmitted through activation of the central complement components 3 and 5 (C3/C5), with concomitant formation of cell surface bound opsonins C3b/iC3b/C3dg and soluble chemotactic anaphylatoxins C3a and C5a, that can attract and activate various types of nucleated effector cells, such as T, NK, B cells and various phagocytes (e.g. PMNs, monocytes, macrophages and dendritic cells). (D) Considering the cellular component, as shown in Figures 1 and 2 , MSC possess very potent immunoregulatory features to modulate adaptive and humoral branches of cellular immunity, that may however be compromised or skewed in an unfavorable direction if IBMIR-mediated killing of infused cells occurs to rapidly for the cells to exert their beneficial effects (e.g. induction of cellular humoral response and alloimunization in response to third-party cells). Overall, the triggering of IBMIR by infused therapeutic cell products may lead to coagulation, complement, and platelet activation, fibrin-cross-linking, and clot formation, with concomitant effector cell activation, and consecutive MSC damage and embolization in the microvasculature.