Figure 5.
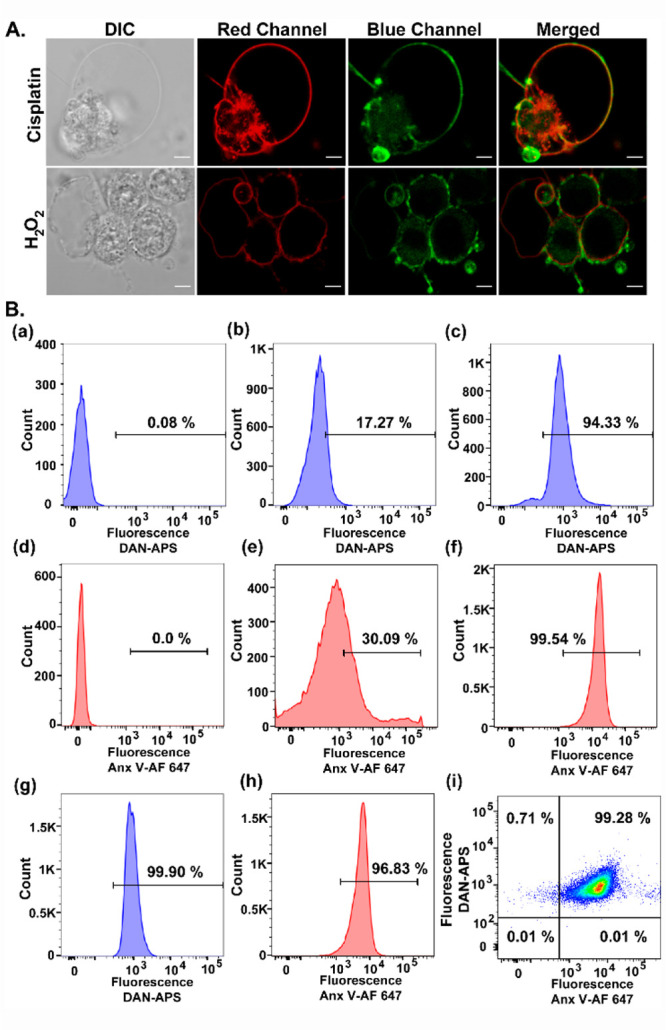
(A) Representative confocal single z-plane images of apoptotic HeLa cells incubated with Anx V-AF 647 (15 min) and DAN-APS (7 μM, 15 min) at 25 °C in buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2) and washed. For inducing apoptosis, HeLa cells were incubated with cisplatin (20 μM, 24 h) or H2O2 (0.2 mM, 4 h) in serum-free DMEM media. The cells were irradiated with λex of 633 nm and a two photon laser (λex: 780 nm), and fluorescence emissions at the red channel (λem: 650–700 nm) and blue channel (λem: 420–460 nm) were collected successively. DIC images in left column. Scale bar, 5 μm. (B) Representative flow cytometry results indicating the effectiveness of DAN-APS for the detection of both viable and apoptotic cells. Cells were treated with either DAN-APS (7 μM, 15 min) (b, c) or Anx V-AF 647 (e, f) or both DAN-APS and Anx V-AF 647 (g, h). Data for probe untreated control cells are shown (a) and (d). All measurements were performed at 25 °C in 10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2. For flow cytometry experiments, the following excitation sources/emission filters were used: Anx V-AF 647, 640 nm/670 nm; DAN-APS, 405 nm/450 nm. Cell count versus mean fluorescence intensities of either DAN-APS or Anx V-AF 647 are plotted as histograms (a–h). (i) Scatter plot of apoptotic cells treated with both DAN-APS and Anx V-AF 647.
