Figure 2.
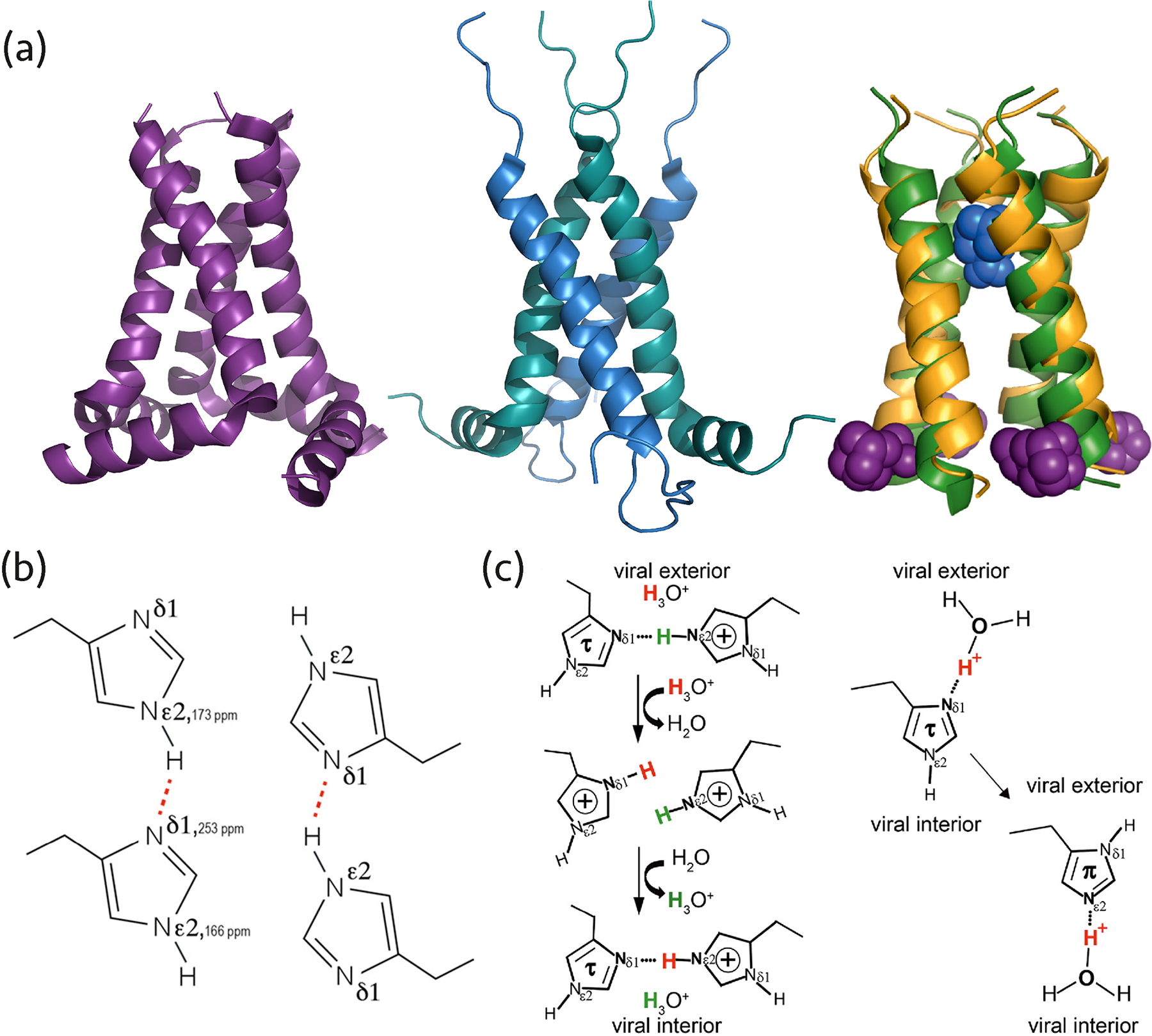
(a) AM2 structures solved by MAS NMR: left, homotetramer (PDB: 2L0J), middle, dimer of dimers (PDB: 2N70), right, superposition of AMT-bound AM2 (PDB: 2KQT) and RMT-bound AM2 (PDB: 2RLF) structures determined by MAS NMR (colored yellow/blue) and solution NMR (colored green/purple). (b) The dimeric arrangement of His-37 in the AM2 pore occurring due to hydrogen bonding, corresponding to the dimer-of-dimers organization of the M2 helices. Reprinted with permission from reference (24). Copyright 2020 American Chemical Society. (c) Two proton conduction models for AM2 His-37 involve different histidine tautomers, protonation states, and hydrogen bonds. One model entails hydrogen bond formation between two histidine residues (left), the other suggests proton shuttling by histidine tautomerization and hydrogen bonding with hydronium ions (right). Reprinted with permission from reference (25). Copyright 2020 American Chemical Society.
