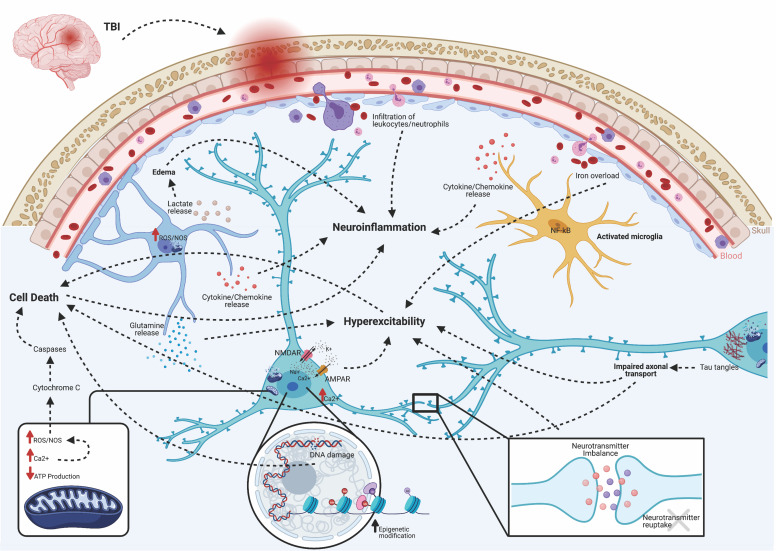
Fig. 2.
Acute pathologies of post-traumatic epileptogenesis. Brain injury triggers several acute pathologies. Direct insult compromises the blood-brain barrier, allowing infiltration of peripherally circulating immune cells, such as leukocytes, macrophages, and neutrophils. NF-κB translocates to the nuclei of microglia, transforming them to an activated phenotype. This induces cellular proliferation and the release of inflammatory amplifiers, such as chemokines, cytokines, reactive oxygen species (ROS), and nitric oxide synthase (NOS). Macrophages participate in the cleanup of damaged cells and debris, but based on their functional activation state, may either exacerbate damage or initiate repair mechanisms. Lactate release from astrocytes contributes to water retention and edema. Excess iron from a leaky BBB can contribute to hyperexcitability. Excessive accumulation of glutamate and aspartate neurotransmitters due to spillage from damaged neurons or impaired reuptake by astrocytes activates NMDA and AMPA receptors located on postsynaptic membranes, allowing for influx of calcium ions. Together with the release of Ca2+ stores from the endoplasmic reticulum, increases in Ca2+ leads to production of ROS and activation of calpains. Damaged or dysfunctional mitochondria create a deficit of available ATP, leading to Na+/K+ pump failure, activation of Ca2+ channels, and further production of ROS/NOS. Cytochrome C released into the cytosol activates cell death pathways via caspase proteins. Epigenetic modifications, in the form of increased HDAC activity and altered DNA/histone methylation, changes transcriptionally active sites, including many genes associated with hyperexcitability and serotonin-to-melatonin conversion. Furthermore, DNA damage leads to apoptosis and cell loss. Progressive axonal damage and tau tangles lead to impaired axonal transport and results in both neurodegeneration and hyperexcitability. Together, these acute pathologies are both adaptive and maladaptive. The former contributes to functional and beneficial recovery, whereas the latter exacerbates epileptogenesis and the progression of abnormal electrographic activity.