Broussard and Green highlight work from Prechova et al. that identifies plectin as a mechanical integrator of cytoskeletal and adhesive networks for cellular tensional homeostasis.
Abstract
The integration of cytoskeletal/adhesive networks is critical to epithelial mechanobiology. In this issue, Prechova et al. (2022. J. Cell Biol. https://doi.org/10.1083/jcb.202105146) demonstrate that the cytolinker protein plectin is essential for the construction of a cortical cytoskeletal architecture required for epithelial tensional homeostasis.
The ability to generate, sense, and respond to mechanical stimuli is of fundamental importance in cell biology. Mechanical forces are translated into biological outcomes through a process termed mechanotransduction to regulate essentially all types of cell behaviors ranging from proliferation, differentiation, motility, and apoptosis. Many pathological conditions have etiologies involving mechanical signaling, including skin, gut, lung, and cardiovascular diseases as well as cancer progression and metastasis. This process depends on cooperation of an integrated network of cytoskeletal filaments that are anchored at both cell–substrate and cell–cell interfaces. In epithelial cells, the actin cytoskeleton forms a cortical belt associated with adherens junctions that is critical for force generation and tissue morphogenesis. The intermediate filament (IF) cytoskeleton, which comprises keratins among other IF proteins, acquires a rim-and-spoke arrangement, with prominent radial filaments linking the nuclear and plasma membrane compartments and a less conspicuous cortical ring, both anchored at desmosomal junctions (1). How these two IF arrangements organize as well as their relative contributions to F-actin–dependent cell mechanics and mechanosignaling are not well understood.
Cytolinker proteins of the plakin family mediate physical linkages of diverse cytoskeletal systems and are thus potential candidates to facilitate crosslinking mechanisms. Plectin is one such cytolinker, containing an N-terminal canonical actin-binding domain (ABD) linked to a C-terminal IF-binding domain (IFBD) through a central rod domain. Loss-of-function mutations in the human gene encoding plectin lead to structural failure in tissues undergoing large amounts of mechanical strain, resulting in autosomal recessive muscular dystrophy associated with skin blistering (epidermolysis bullosa simplex; 2). While the cellular functions of plectin in cell–substrate adhesion are well studied, its roles in cell–cell adhesion remain less clear. In this issue, Prechova et al. identify a new function for plectin in organizing the architecture of the cortical cytoskeletal and cell–cell junctional networks, specifically through coupling the cortical actin belt and the keratin rim (3).
The authors first assessed the consequences of ablating plectin in epithelial sheets through either CRISPR/Cas9-mediated genetic depletion or the use of a high-affinity plectin ligand inhibitor called plecstatin-1. Plectin inactivation profoundly disrupted the rim-and-spoke organization of the IF cytoskeleton, resulting in sparse, thickened radial spokes that were anchored at less dense and misshapen desmosomes (Fig. 1). Moreover, super-resolution fluorescence microscopy and transmission EM revealed a loss of the keratin rim and a cortical actin belt that remained intact, suggesting that plectin is essential for forming the circumferential IF arrangement at the cell cortex. Plectin localized to cell–cell borders and was often found colocalizing with the desmosomal protein desmoplakin (DSP). While plectin has been shown to interact with immunoprecipitated DSP in vitro (4), it is unknown if this interaction is required for plectin junctional localization. Instead, Prechova et al. showed that the ABD of plectin is recruited to cell–cell interfaces through interactions with the cortical actin belt, and rescue experiments demonstrated that both the ABD and IFBD domains of plectin are required for rim formation. Intriguingly, two plectin isoforms implicated in IF organization (P1a and P1f) could restore the formation of the circumferential keratin rim, while isoforms of plectin that are associated with cellular organelles (P1 and P1b) did not facilitate rim architecture, indicating isoform-specific regulation.
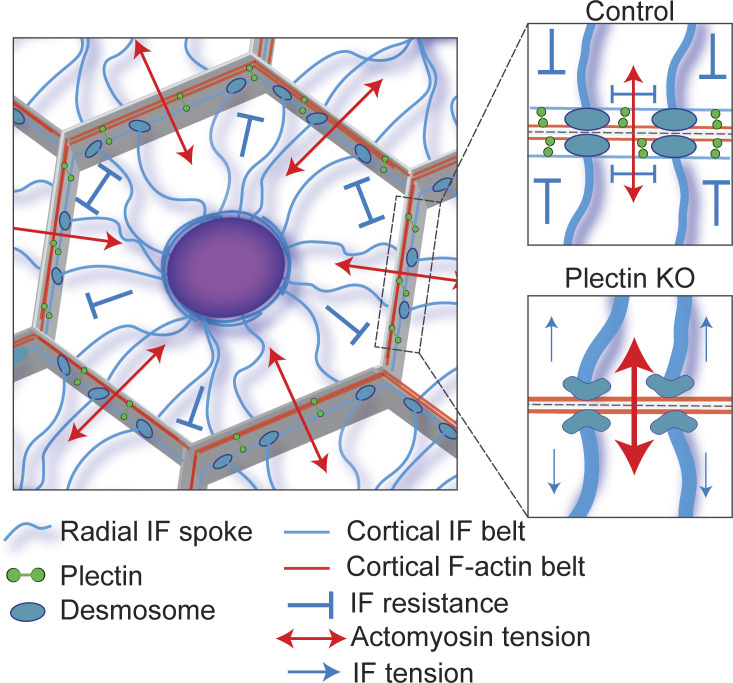
Figure 1.
Model of plectin-mediated cytoskeletal crosstalk at the cell–cell interface. Cells within epithelial tissues are in a state of isometric tension, with actomyosin-generated forces being balanced by the resistive capacity of the IF network. The IF network consists of radial spokes extending from the nucleus and anchored at desmosomes. These spokes are interconnected by a subplasmalemmal circumferential IF rim that is coupled to the cortical actomyosin machinery through plectin, providing mechanical resistance along the plasma membrane. Plectin deficiency results in a loss of the circumferential IF rim and redistribution of cytoskeletal forces. Absence of the IF rim is associated with bundling of IF spokes, increased actomyosin contractility, and aberrant tensile loading of desmosomes.
A key function of the IF cytoskeleton is to impart mechanical resilience to tissues; however, the mechanosensitive nature of the IF system remains unclear. To address this question in the context of plectin deficiency, the researchers used traction force microscopy and FRET-based tension sensors. They showed that plectin localization appears to be sensitive to mechanical stimulation. Epithelial monolayers exposed to cyclic stretch exhibited an enrichment of junctional plectin and an associated reorientation of keratin IF. Furthermore, they observed elevated levels of actomyosin contractility and increased tension on adherens junctions in plectin-deficient cells. Previous work suggests that under steady-state conditions, DSP appears to be under little if any mechanical load (5). Nonetheless, Prechova et al. provided evidence that loss of plectin results in increased tension across DSP, suggesting that loss of cytoskeletal crosslinking perturbs cellular tensional homeostasis (3).
The exact mechanisms by which a single crosslinker can have such wide-ranging effects on cell adhesion and force generation remain elusive. However, work in astrocytes has shown that cytoplasmic vimentin IF are coupled to F-actin arcs through plectin (6). This coupling creates a force balance between retrograde moving contractile F-actin arcs and a network of IF that act to resist this movement. Mutants of the cytolinker DSP that modulate the strength of the desmosome/IF connection have been used to establish that the desmosome/IF network works in cooperation with components of the actin cytoskeleton to tune the mechanical characteristics of cell monolayers (7). Prechova et al. offered additional evidence that the keratin IF rim constitutes a further layer of regulation in balancing contractile and tensile forces among the adhesive/cytoskeletal networks, with actomyosin contributing tension that is resisted by the IF-based network. Since there is tissue-specific variability in IF network composition, this could provide an opportunity to differentially regulate tissue mechanics by balancing and tuning forces among cytoskeletal systems. Indeed, recent studies have shown that the desmosome/IF network is critical to the formation of a mechanically polarized epidermis, orchestrating cell layer–specific functions such as basal cell stratification and formation of the tight junction barrier (8).
The observations described by Prechova et al. raise a number of interesting questions. Plectin localization to the cell cortex requires the cortical actin belt. Does plectin subsequently recruit and anchor preformed IF? Or is it possible that, similar to what has recently been shown for desmosomes (9), plectin serves as a platform for the nucleation of new IF? Moreover, post-translational modifications of plakin family members, such as phosphorylation in the C-terminal IF-binding regions of plectin, govern their interaction with IF, coordinating cytoskeletal dynamics and cellular adhesion (10). Do similar modifications affect the ability of plectin to form the IF rim and/or tune its mechanical properties? What about the IF network itself, which is well known to be heavily post-translationally modified? Understanding the post-translational modifications that modulate the interactions among the adhesive and cytoskeletal machinery may provide researchers with more targeted approaches to study their functions in mechanobiology. Given that mechanical forces are pervasive signals in biology, a better comprehending of how cells build an integrated cytoskeletal and adhesive network may provide insight into a wide range of both homeostatic and pathological conditions.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01 AR041836, R37 AR043380, R01 CA228196, and the J.L. Mayberry Endowment to K.J. Green,. J.A. Broussard was supported by NIH K01 AR075087.
The authors declare no competing financial interests.
References
- 1.Quinlan, R.A., et al. 2017. J. Cell Sci. 10.1242/jcs.202168 [DOI] [Google Scholar]
- 2.Smith, F.J., et al. 1996. Nat. Gen. 10.1038/ng0896-450 [DOI] [Google Scholar]
- 3.Prechova, M., et al. 2022. J. Cell Biol. 10.1083/jcb.202105146 [DOI] [Google Scholar]
- 4.Eger, A., et al. 1997. J. Cell Sci. [DOI] [PubMed] [Google Scholar]
- 5.Price, A.J., et al. 2018. Nat. Commun. 10.1038/s41467-018-07523-0 [DOI] [Google Scholar]
- 6.Jiu, Y., et al. 2015. Cell Rep. 10.1016/j.celrep.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Broussard, J.A., et al. 2017. Mol. Biol. Cell. 10.1091/mbc.E16-07-0520 [DOI] [Google Scholar]
- 8.Broussard, J.A., et al. 2021. Curr. Biol. 10.1016/j.cub.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moch, M., et al. 2020. Cell. Mol. Life Sci. 10.1007/s00018-019-03198-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouameur, J.-E., et al. 2013. J. Cell Sci. 10.1242/jcs.127779 [DOI] [PMC free article] [PubMed] [Google Scholar]