The authors report a modular T-cell control system, CRASH-IT, that allows the function of T cells activated by their TCR or genetically introduced TCRs or CARs to be adjusted in a reversible manner over a wide dynamic range.
Abstract
Adoptive transfer of genetically modified or donor-derived T cells can efficiently eradicate human tumors but is also frequently associated with major toxicity. There are several switches that can be used to kill the infused cell pool in the case of major toxicity, but the irreversible nature of these suicide switches means that the therapeutic effect is lost when they are used. To address this issue, we engineered a small-molecule responsive genetic safety switch that in the absence of drug robustly blocked cytotoxicity and cytokine expression of primary human T cells. Upon administration of drug, T-cell functions were restored in a reversible and titratable manner. We showed that this T-cell switch was universal, as it could be combined with endogenous or transduced T-cell receptors (TCR), as well as chimeric antigen receptors. The modular nature of the Chemically Regulated - SH2-delivered Inhibitory Tail (CRASH-IT) switch concept, in which inhibitory domains are brought to activating immune receptors in a controlled manner, makes it a versatile platform to regulate the activity of cell products that signal through immunoreceptor tyrosine-based activation motif (ITAM)–containing receptors.
Introduction
Over the past few years, a number of adoptive T-cell therapies have been developed for the treatment of both hematologic cancers and solid tumors (1, 2). Donor lymphocyte infusion (DLI) was developed following the discovery of the antitumor effect of donor-derived T cells upon allogeneic hematopoietic stem cell transplantation (3). More recently, T cells modified with chimeric antigen receptors (CAR) and T-cell receptors (TCR) have been utilized to provide patients with tumor-reactive T-cell populations, and the clinical activity of CD19- and BCMA-targeted CAR T cells in patients with hematologic malignancies (4, 5) led to the approval of these T-cell products for B-cell acute lymphoblastic leukemia, diffuse large B-cell lymphoma, and multiple myeloma.
Broader applicability of adoptive T-cell therapies in cancer is presently limited by safety concerns. Specifically, on-target but off-tumor toxicity is often observed when the antigens that are targeted by the adoptively transferred T-cell pool are also expressed in healthy tissue (6). Although substantial effort has been made to discover truly cancer-specific markers, strictly cancer-specific markers that are expressed by tumors of a large fraction of patients have proven extremely rare. In addition, unexpected cross-reactivity of genetically modified T cells with self-antigens that show low-level expression in vital tissues is associated with severe toxicity (7). Finally, even when fully tumor-specific activation of the infused cells is achieved, cytokine release upon T-cell recognition of a large tumor mass can form a safety concern (8), and strategies to tune the strength of T-cell activation are actively being sought.
The substantial risk of treatment-induced toxicity upon adoptive T-cell therapy led a number of groups to develop genetically encoded suicide switches, such as the HSV-TK, iCas9, and CD20-based cell-surface marker suicide switches, which can be triggered by drug administration or antibody administration at the moment that major toxicity is observed (9). Such suicide switches have primarily found use in the setting of DLI. However, the binary nature of these switches does not allow a titration of T-cell functions and these systems have found limited use in the context of TCR/CAR-modified T cells. Emerging evidence suggests the tyrosine kinase inhibitor dasatinib can be used to control the function of CAR T cells (10), but with the caveat that the activity of endogenous T cells reactive against, for instance, persisting herpesviruses or tumor antigens is also abrogated.
In additional work, safety switch technologies that can reversibly control CAR T cells have been engineered (11,12,13). Fusion of CARs with protein stability control domains allows creation of small-molecule mediated OFF switches (14,15,16), and incorporation of the PD1 signaling domain in CAR designs is used to repurpose the CARs as NOT gates (17). However, a small-molecule regulated generic ON switch that does not require covalent modification of the antigen receptor, and hence can be combined with any TCR or CAR, has not been reported to our knowledge.
Materials and Methods
Retroviral DNA constructs
All DNA constructs were generated in the MP71 retroviral expression vector backbone (a kind gift from Christopher Baum, Hannover Medical School, Hannover, Germany; ref. 18). In brief, codon-optimized DNA sequences were synthesized by Integrated DNA Technologies, Inc. as gene fragments and cloned into the MP71 vector by Gibson assembly using the Gibson Assembly Master Mix according to the manufacturer's instructions (NEB, catalog no. E2611L; ref. 19). Detailed description of the Chemically Regulated - SH2-delivered Inhibitory Tail (CRASH-IT) switch variants and the codon-optimized DNA sequences encoding switch molecules were given in Supplementary Table S1. Genetically engineered TCR and CAR constructs were expressed from a separate plasmid. A second-generation CD19-specific CAR (20) was subcloned into the MP71 backbone from the SFG-19-28z vector in an IRES-truncated human EGFR (huEGFRt) reporter (21) configuration by Gibson assembly. The HLA class I–restricted CDK4 TCR (TCR 17) and the HLA class I–restricted NY-ESO-1 TCR (TCR 1) have been described previously (22, 23). The variable domain sequences of the HLA class II–restricted CMV-pp65 TCR (24) were kindly provided by M.H. Heemskerk (LUMC, Leiden, the Netherlands) and were cloned into the TCR flex MP71 vector (23).
Cell lines and cell culture
FLYRD18 (in-house), T2 (in-house), K562 (in-house), Raji (in-house), NKIRTIL006 (in-house; ref. 25), MM90904 (a kind gift from Marco Donia in 2012, Herlev Hospital, Herlev, Denmark), Mel526 (a kind gift from Steven Rosenberg in 2006, NIH, Bethesda, MD; ref. 26), Daudi (received in 2018 from ATCC, catalog no. CCL-213) and CBH 5477 (a kind gift from M.H. Heemskerk in 2018, LUMC, Leiden, the Netherlands) cells were cultured in Iscove's modified Dulbecco's medium (IMDM; Thermo Fisher Scientific, catalog no. 21980065), 8% FCS (Sigma-Aldrich, catalog no. F7524-500ML), and penicillin-streptomycin (100 IU/mL penicillin, 100 μg/mL streptomycin, Sigma-Aldrich, catalog no. 11074440001), which was considered standard culture medium. FLYRD18, MM90904, Mel526, and NKIRTIL006 cells were passaged every 2 to 3 days with trypsin-EDTA (Thermo Fisher Scientific, catalog no. 15400054). All cell lines were tested for Mycoplasma using PCR-based screening using primers described previously (27), and they were found to be negative. Cell lines were authenticated using the short tandem repeat (STR) method in the past year according to the instructions from the manufacturer of the kit (Promega, catalog no. DC6530) by comparison with reference sequences in ATCC database where available, or the STR profiles from the earliest available record. Passage numbers are kept at minimum by using frozen stocks from earliest possible date available and culturing cells from these stocks for up to 10 passages.
Retrovirus production
Retroviral particles were produced in FLYRD18 packaging cells. In brief, 700,000 FLYRD18 packaging cells were plated per 10 cm dish in standard culture medium 1 day prior to transfection. The next day, cell culture medium was refreshed with IMDM supplemented with 8% FCS without antibiotics. A total of 25 μL of X-tremeGENE 9 (Roche, catalog no. 6365809001) was mixed with 800 μL Opti-MEM (Thermo Fisher Scientific, catalog no. 11058-021) and incubated at room temperature for 5 minutes. Subsequently, the Optimem-X-tremeGENE 9 mixture was added on top of 1:1 mixture of two retroviral plasmids that separately encoding indicated antigen receptor and CRASH-IT switch constructs (10 μg total DNA) dissolved in water and incubated for 15 minutes, and the resulting transfection mixture was added dropwise onto the packaging cells. The supernatant containing retrovirus was harvested 48 hours after transfection and immediately used or snap-frozen in liquid nitrogen.
T-cell isolation and activation
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats from healthy donors (Sanquin) by Ficoll-Isopaque density centrifugation at 500 × g (GE Healthcare, catalog no. 17-1440-03; ref. 23) and were stored frozen until further use. To generate activated T-cell populations, PBMCs were thawed in PBS containing 5% FCS, counted, and mixed with CD3/CD28 Dynabeads (Cell Signaling Technology, catalog no. 40203D) at a 1:1 cell:bead ratio, at a density of 1 × 107 cells/mL. Following a 30-minute incubation at room temperature on a tumbler, the mixture was put on a magnet and unbound cells were removed. Bead-bound T cells were resuspended in RPMI (Thermo Fisher Scientific, catalog no. 11875-093), 10% human serum (Sigma-Aldrich, catalog no. H3667-100ML), penicillin-streptomycin containing 100 IU/mL IL2 (Novartis, catalog no. 05060229220264) and 5 ng/mL IL15 (Peprotech, catalog no. 200-15), and plated at a density of 0.75 × 106 cells/mL.
Spin-transduction of T cells
Twenty-four–well nontreated cell culture plates were covered with 10μg/mL retronectin (Takara, catalog no. T100B) overnight at 4°C. The next day, the retronectin solution was removed and wells were blocked with 2% BSA (Sigma-Aldrich, catalog no. A9418-500g) in PBS for 30 minutes. Activated T cells (0.25 × 106 cells/mL in RPMI, 10% human serum, penicillin-streptomycin, 200 IU/mL IL2 and 10 ng/mL IL15) were then mixed with retroviral supernatant at a 1:1 ratio (volume:volume) in retronectin coated 24-well plates and centrifuged at 2,000 RPM for 90 minutes at room temperature.
Sorting and rapid expansion of T cells
Culture medium of CRASH-IT switch–expressing cells was supplemented with 0.5 μmol/L dTAG-13 PROTAC (Tocris, catalog no. 6605) or 10 μmol/L asunaprevir (MedChemExpress, catalog no. HY-14434), starting 1 day before cell sorting until 4 days before 51Cr assays. Primary human T cells modified with the CDK4 TCR plus either the CRASH-IT switch or IRES-EGFP vector control were sorted on a Beckman Coulter Moflo Astrios. Cells were cultured in RPMI, 10% human serum, penicillin-streptomycin, 100 IU/mL IL2 and 5 ng/mL IL15 plus 0.5 μmol/L dTAG-13 PROTAC or 10 μmol/L asunaprevir for 7 days. After this, cells were expanded using a rapid expansion protocol (REP). In brief, a mixture of 2 × 108 feeder cells (PBMCs from three donors isolated by Ficoll-Isopaque density centrifugation) was generated by irradiation at 4,000 rad (Gammacell 40 Exactor) and resulting feeder cells were then mixed with 1 × 106 sorted T cells, and 4.5 μg OKT3 (Invitrogen, catalog no. 16-0037-85), IL2 (3,000 IU/mL) in 150 mL 20/80 T-cell Mixed Media (Thermo Fisher Scientific, catalog no. 041-96658P), penicillin-streptomycin (Invitrogen), and 0.5 μmol/L dTAG-13 PROTAC or 10 μmol/L asunaprevir. At day 6, culture medium was refreshed, and cultures were subsequently split into two every 3 days with fresh medium. At day 12, cells were switched to standard T-cell culturing conditions (RPMI, 10% human serum, penicillin-streptomycin, 100 IU/mL IL2, and 5 ng/mL IL15) for 4 to 8 days, until use in 51Cr assays.
Peptide loading
For use as T-cell targets, T2 and CBH 5477 cells were loaded with the indicated concentrations of HLA-A*02:01 restricted mutant CDK4 peptide (ALDPHSGHFV), HLA A*02:01 restricted NY-ESO-1 peptide (SLLMWITQA), or HLA-DR1–restricted CMV peptide (KYQEFFWDANDIYRI) in IMDM for 1 hour at 37°C. Cells were washed once and used in coculture experiments. All peptides are kind gifts from Huib Ovaa, Leiden University, Leiden, the Netherlands).
Activation of T cells with immobilized anti-CD3 or anti-CD3/CD28
Ninety-six–well plates were coated with 1:200 diluted (in PBS) anti-CD3 (eBioscience, catalog no. 16-0037-85) or anti-CD3/CD28 (eBioscience, catalog no. 16-0289-85) overnight. Subsequently, antibody solution was removed and plates were washed twice with PBS before cells were plated to the antibody-coated plates for activation of endogenous TCR complexes.
Cytokine release assay and antibody staining
T cells were pretreated with the indicated concentrations of asunaprevir, the FDA-approved HCV NS3/4A inhibitor grazoprevir (MedChemExpress, catalog no. HY-15298), dTAG-13 PROTAC or DMSO control in T-cell medium (RPMI, 10% human serum, penicillin-streptomycin, 100 IU/mL IL2, and 5 ng/mL IL15) for 24 hours before coculture experiments. A total of 100,000 T cells and 100,000 of the indicated tumor cells were mixed in T-cell medium supplemented with golgi-plug (1:1,000 dilution, BD Biosciences, catalog no. 51-2301KZ) and anti-LAMP1-APC (1:100 dilution, BioLegend, catalog no. 328620) in the presence of the indicated drugs or DMSO control in 96-well plates and incubated for 5 hours at 37°C. After incubation, cells were washed once with PBS and stained with IR dye (Invitrogen, catalog no. L34976) at 1:400 dilution for 5 minutes at 4°C. Subsequently, cells were washed once with FACS buffer (PBS plus 0.5% BSA) and stained with anti-CD8-PerCP Cy5.5 (1:20 dilution, BD Biosciences, catalog no. 341050), anti-CD4 BV711 (1:50 dilution, BioLegend, catalog no. 317440), anti-murine constant TCR-PE (in experiments using TCR-transduced T cells, 1:200 dilution, BD Biosciences, catalog no. 553172) or cetuximab-PE (in experiments using CAR-transduced T cells that expressed the huEGFRt, 1:200 dilution, R&D Systems, catalog no. FAB9577P) for 20 minutes at 4°C. Cells were then washed once with FACS buffer and fixed with BD fixation and permeabilization solution (catalog no. 51-2090KZ) for 20 minutes at 4°C. Following permeabilization, cells were washed twice with BD perm/wash buffer (catalog no. 51-2091KZ) and stained with anti-IFNγ-BV421 (1:100 dilution, BD Biosciences, catalog no. 564791), anti-IL2-PE-Cy7 (1:100 dilution, BD Biosciences, catalog no. 560707), and anti-TNFα-BV650 (1:100 dilution, BioLegend, catalog no. 502938) diluted in perm/wash buffer for 20 minutes at 4°C. Cells were then washed twice, resuspended in 100 μL FACS buffer and analyzed directly on a Fortessa Special Order analyzer. For the detection of HA-tag fusion proteins, T cells were intracellularly stained with anti-HA-AF647 (1:200 dilution, Cell Signaling Technology, catalog no. 3444S). For the detection of CD19 cell-surface expression, K562, Raji, and Daudi tumor cells were cell surface stained with anti-CD19-PE (1:200 dilution, BD Biosciences, catalog no. 345789) or isotype control (BioLegend, catalog no. 400111), using the same staining protocol.
51Cr assays
One day prior to 51Cr assays, T cells were pretreated with 0.5 μmol/L dTAG-13 PROTAC, 10 μmol/L asunaprevir or DMSO in standard T-cell culturing conditions (RPMI, 10% human serum, penicillin-streptomycin/100 IU/mL IL2, and 5 ng/mL IL15). A total of 5 × 105 tumor cells were resuspended in 100 μL media, mixed gently with 100 μCi 51Cr (PerkinElmer, catalog no. NEZ030002MC) and then incubated at 37°C for 45 minutes. In parallel, 100 μL of dilutions of T cells treated with either dTAG-13 PROTAC, asunaprevir or DMSO control were aliquoted into 96-well plates. 100 μL medium only (spontaneous release) and 100 μL 1% Triton solution (maximal release) were used as controls. After labeling, target cells were washed, resuspended at 50,000 cells/mL, and added to the 96-well plates at 100 μL per well. Plates were incubated at 37°C for 4 hours, and counts in supernatant were determined using TopCount NXT(PerkinElmer). Experimental values were normalized using spontaneous and maximal release controls.
Statistical analyses
Flow cytometry data were analyzed with FlowJo 10.7.1 software and data visualization and statistical analyses were performed using GraphPad Prism 8.4.3 software.
Results and Discussion
To develop a system that can reversibly control the activity of infused T-cell pools that express any antigen receptor of interest, we hypothesized that we would need a strategy in which an inhibitory signal is brought to the activating (e.g., CAR or TCR) signaling complex in a titratable manner. A shared property of CARs and TCRs is that signaling through these receptors leads to the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAM) and the subsequent recruitment of the Zap70 kinase, which binds to the phosphorylated ITAMs via its SH2 domains. We postulated that if signaling domains derived from inhibitory receptors could be shuttled to activated antigen receptors by fusion to the Zap70 SH2 domains, this would attenuate TCR/CAR signaling. To provide proof of concept for SH2 domain–assisted inhibitory tail delivery, we focused on the PD1 intracellular domain, because the inhibitory effect of PD1 signaling on cytokine production and cytotoxicity is reversible (28) and the physical proximity of PD1 to TCR microclusters is crucial for T-cell suppression (29). Therefore, we generated a Zap70 2×SH2 domain-PD1 tail fusion protein (from here on abbreviated as Zap70-PD1), with the aim to bring the inhibitory domain of PD1 in close proximity to the TCR complex (Fig. 1A and B).
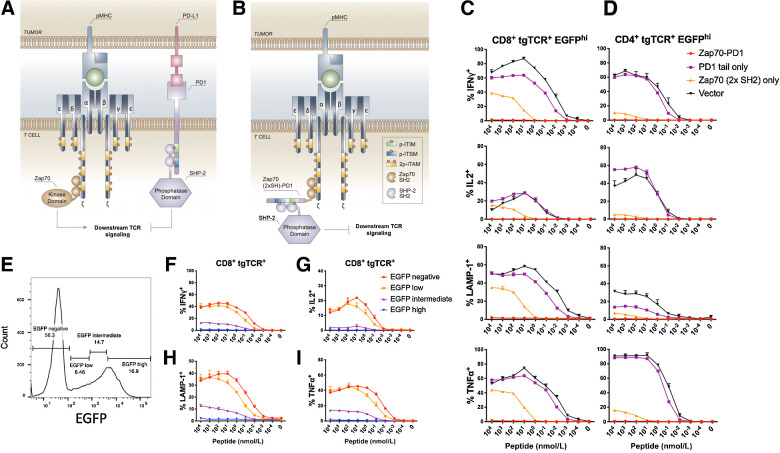
Figure 1.
Efficient suppression of T-cell function by Zap70 SH2–mediated PD1 tail recruitment. A, Schematic diagram of early steps in the TCR signal transduction pathway. B, Schematic diagram of Zap70 SH2–mediated recruitment of the PD1 tail to the activated TCR complex and resulting inhibition of TCR signaling. ITIM, immunoreceptor tyrosine-based inhibitory motif; ITSM, immunoreceptor tyrosine-based switch motif. C and D, Primary human T cells modified with the MHC class I–restricted CDK4 TCR and either Zap70-PD1, Zap70 2×SH2 domains, PD1 tail, or vector control were cocultured with CDK4 peptide–loaded T2 cells. Data depict IFNγ, IL2, TNFα production, and cell-surface LAMP1 expression of CDK4 TCR+ EGFP-high CD8+ (C) and CD4+ (D) T cells. E, Gates used to define EGFP-high, -intermediate, -low, and -negative populations of CDK4 TCR+ T cells modified with the Zap70-PD1-IRES-EGFP vector. F–I, Zap70-PD1-IRES-EGFP–expressing T cells from the dataset as in C analyzed using the EGFP expression gates in E. Error bars represent SD (n = 3). Data are representative of two independent experiments.
To assess the effect of Zap70-PD1 on T-cell activation, primary human T cells were transduced with a high-affinity CDK4 neoantigen-specific MHC class I–restricted TCR (22), together with either the Zap70-PD1 fusion, the Zap70 SH2 domains without inhibitory tail (Zap70 2×SH2), the PD1 intracellular domain (PD1 tail), or a vector control. Analysis of T-cell cytokine production (IFNγ, IL2, and TNFα) and T-cell degranulation (LAMP-1 cell-surface expression) upon incubation with T2 target cells loaded with the CDK4 neoantigen demonstrated that expression of the free PD1 tail did not substantially alter T-cell functionality (reduction in percentage IFNγ+ cells at 10 nmol/L peptide concentration relative to vector control: 1.4-fold, P = 0.000049; Fig. 1C and D). Expression of the Zap70 2×SH2 domains without the PD1 tail resulted in a modest inhibition of T-cell functions (reduction in percentage IFNγ+ cells at 10 nmol/L peptide concentration relative to vector control: 5.8-fold, P < 0.000001; Fig. 1C and D) that may be explained by competition with wild-type Zap70 (30). When the intracellular domain of PD1 was linked to the Zap70 SH2 domains, a highly efficient suppression of both T-cell cytokine production and T-cell degranulation was observed (reduction in percentage responding cells at 10 nmol/L peptide concentration: IFNγ: 104-fold; IL2: 110-fold; TNFα: 81-fold; cell surface LAMP1: 40-fold, all relative to vector control; Fig. 1C and D). Inhibition of T-cell functionality was both observed for CD8+ and CD4+ T cells. Furthermore, comparison of the degree of inhibition of T-cell activity in gene-modified cell populations with different levels of EGFP reporter expression revealed that the level of Zap70-PD1 expression correlated with inhibitory activity (Fig. 1E–I), suggesting that regulation of Zap70-PD1 protein levels could be used to modulate T-cell function.
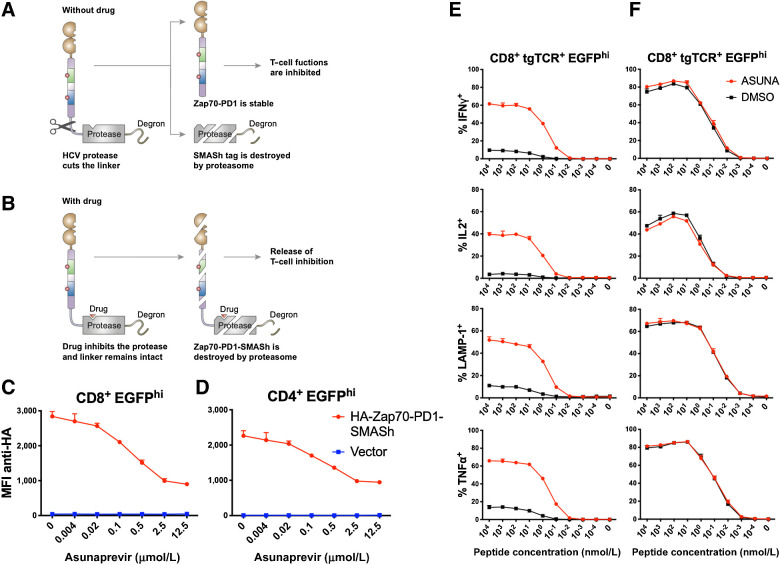
To achieve pharmacologic control over Zap70-PD1 protein levels, we subsequently generated fusion proteins with the small molecule–assisted shutoff (SMASh) tag (Fig. 2A and B; ref. 31), which consisted of the HCV NS3/4A protease plus a degron that results in rapid protein degradation by the proteasome. In the absence of a hepatitis C virus (HCV) protease inhibitor, the HCV protease cleaves the linker between the protein of interest and the degron, thereby preventing protein degradation. In the presence of a protease inhibitor, the full-length fusion protein is degraded by the proteasome. To test whether the level of Zap70-PD1 fusion protein could be controlled in this manner, we introduced an N-terminal HA-tagged Zap70-PD1-SMASh fusion protein into human T cells. Analysis of fusion protein levels by intracellular staining revealed that inhibition of protease activity by the HCV NS3/4A protease inhibitor asunaprevir resulted in a reduction in fusion protein level in primary human CD8+ and CD4+ cells, with half-maximal inhibition observed around 0.2 μmol/L (Fig. 2C and D). The Zap70-PD1-SMASh fusion protein retained the capacity of the Zap70-PD1 fusion protein to prevent T-cell activation, and this inhibition of T-cell function was reverted by the addition of asunaprevir for both primary human CD8+ T cells (Fig. 2E) and CD4+ T cells (Supplementary Fig. S1A). Specifically, relative to DMSO-treated cells, CD8+ T-cell activation by peptide-loaded target cells (10 nmol/L) was boosted 8.8×, 11.5×, 6.3×, and 6.6×-fold for IFNγ, IL2, TNFα and cell surface LAMP1 expression, respectively. As a control, the effector function of T cell negative for the Zap70-PD1-SMASh fusion protein was unaltered by protease inhibitor (Fig. 2F; Supplementary Fig. S1B). The effect of asunaprevir on the function of Zap70-PD1-SMASh–modified T cells was profound (Fig. 2E), even though its effects on fusion protein levels were modest (Fig. 2C), suggesting that the signal amplification properties of the TCR signaling pathway make it highly sensitive to signal strength.
Figure 2.
Control of T-cell function by CRASH-IT. A and B, Schematic diagram of the Zap70-PD1-SMASh fusion protein in the absence (A) or presence (B) of asunaprevir. C and D, Intracellular HA staining of primary human T cells modified with an N-terminal HA-tagged Zap70-PD1-SMASh construct or vector control. Effect of prior asunaprevir exposure on HA signal is depicted for EGFP-high CD8+ (C) and CD4+ (D) T cells. MFI, mean fluorescence intensity. E and F, Primary human T cells modified with the MHC class I–restricted CDK4 TCR and either the Zap70-PD1-SMASh construct (E) or vector control (F) were pretreated with 10 μmol/L asunaprevir (ASUNA) or DMSO control. Data depict intracellular IFNγ, IL2, TNFα and cell-surface LAMP1 expression of CDK4 TCR+, EGFP-high, CD8+ T cells upon coculture with CDK4 peptide–loaded T2 cells in the continued presence or absence of asunaprevir. Error bars represent SD (n = 3). Data are representative of two independent experiments.
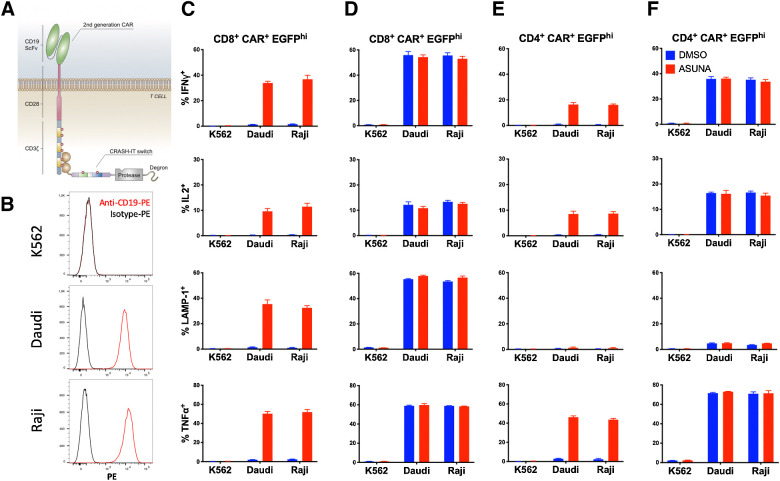
We next tested the flexibility of the CRASH-IT switch system, of which the Zap70-PD1-SMASh construct was the first embodiment, using T cells that were either modified with a second-generation CAR or an intermediate affinity MHC class I–restricted TCR. When human T cells modified with the anti-CD19-CD28-CD3ζ chain CAR (20) were cocultured with CD19+ Raji or Daudi cells, (Fig. 3) cytokine production and degranulation of the CAR T cells was efficiently suppressed by the Zap70-PD1-SMASh construct, and recovered upon drug addition. Likewise, the functional activity of CD8+ T cells modified with a TCR specific for NY-ESO-1 (23) was prevented by the CRASH-IT switch and restored by addition of asunaprevir (Supplementary Fig. S2).
Figure 3.
Control of CAR T-cell function by CRASH-IT. A, Schematic diagram of second-generation anti-CD19-CD28-CD3ζ CAR interacting with the Zap70-PD1-SMASh construct. B, CD19 expression on K562, Daudi, and Raji tumors. Cells were stained with anti-CD19-PE (red line) or isotype control-PE (black line). C–F, Primary human T cells modified with the anti-CD19-CD28-CD3ζ CAR and either Zap70-PD1-SMASh (C and E), or vector control (D and F) were pretreated with 10 μmol/L asunaprevir (ASUNA) or DMSO control. Data depict intracellular IFNγ, IL2, TNFα and cell-surface LAMP1 expression of CAR+, EGFP-high, CD8+ (C and D) and CD4+ (E and F) T cells upon coculture with CD19− K562 tumor cells, or CD19+ Daudi or Raji tumor cells in the continued presence or absence of asunaprevir. Error bars represent SD (n = 3). Data are representative of two independent experiments.
A safety switch used in adoptive T-cell therapies should ideally be titratable and reversible. A dose-dependent recovery of T-cell functionality was demonstrated by analyzing the effect of increasing asunaprevir concentrations in cocultures of antigen-loaded target cells and CDK4-specific T cells modified with the Zap70-PD1-SMASh construct (Supplementary Fig. S3A–S3C), revealing that T cells can be tuned to reach a desired antigen sensitivity.
To understand whether the CRASH-IT platform could act as a reversible regulator of T cells, to switch them from an active to an inactive state and back, asunaprevir-treated and control-treated T cells were washed and cultured for 72 hours in the absence of drug. Subsequently, T cells were again treated with asunaprevir or left untreated and were then exposed to antigen loaded target cells (experimental scheme in Supplementary Fig. S3A). Zap70-PD1-SMASh–modified T cells showed substantial activity only when exposed to asunaprevir during the time of tumor coculture, regardless of whether the cells had previously been exposed to asunaprevir or not. In other words, prior triggering of the CRASH-IT switch did not alter outcome during subsequent use, demonstrating reversibility of the platform (Supplementary Fig. S3D). Small molecule–induced recovery of T-cell function was also demonstrated in coculture experiments using the Mel526 and NKIRTIL006 melanoma cells, which endogenously express the mutant CDK4 neoantigen, and control MM90904 melanoma cells, which express the wild-type CDK4 gene (Supplementary Fig. S4). To characterize the protein domains required to achieve reversible T-cell inhibition, we compared SMASh-tagged versions of Zap70 2×SH2 and Zap70-PD1, and also a SMASh-tagged version of Zap70-PD1 that lacked the degron domain (Supplementary Fig. S4A–S4D). As was observed for protein switches that lacked the SMASh domain (Fig. 1C and D), the presence of the PD1 tail was essential to achieve tight control of TCR signaling, and restoration of T-cell activity upon triggering of the CRASH-IT switch required the presence of the degron that induces proteasomal degradation.
The efficiency of asunaprevir-induced protein degradation was higher in CD8+ than CD4+ cells (Fig. 2C and D). Potentially because of this, asunaprevir-induced restoration of T-cell function was more profound for CD8+ T cells than for CD4+ T cells (Supplementary Fig. S2; Supplementary Fig. S3B and S3C). To explore the feasibility of creating variant CRASH-IT switch systems with an optimized dynamic range for different immune-cell types, we created a modified version of Zap70-PD1-SMASh (Supplementary Fig. S5A) that showed a slightly less stringent T-cell suppression in the absence of asunaprevir in CD8+ cells. This tuned Zap70-PD1-SMASh (tuZap70-PD1-SMASh) switch retained the capacity to efficiently suppress the function of CD4+ T cells modified with the CDK4 TCR, and recovery of T-cell functions upon asunaprevir addition was substantially improved (Supplementary Fig. S5B). To test whether this switch system could also be used to control CD4+ T-cell activation at the moment the CD4 coreceptor was coengaged, we cotransduced primary human CD4+ T cells with the tuZap70-PD1-SMASh construct and an HLA class II–restricted CMV-specific TCR, and co-cultured these cells with peptide-loaded CBH 5477 cells. Antigen sensitivity was reduced by approximately 1,000-fold by introduction of the tuned Zap70-PD1-SMASh construct, and asunaprevir treatment resulted in a near-complete recovery of CD4+ T-cell functions (Supplementary Fig. S5C and S5D), demonstrating how optimized CRASH-IT systems can be created for specific cell types.
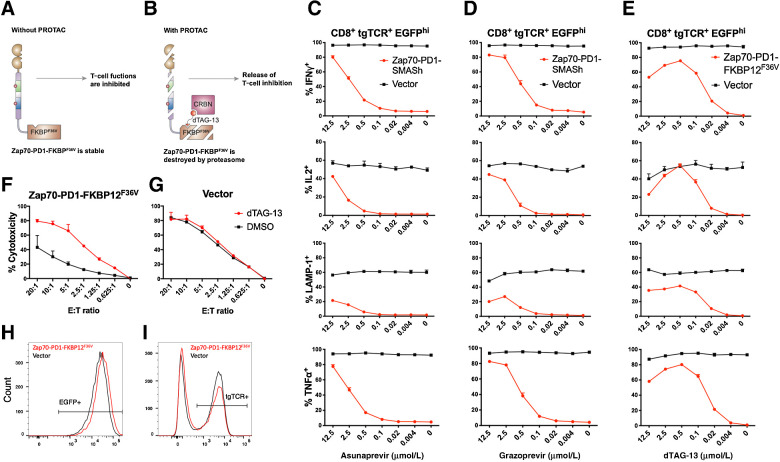
A central aspect of CRASH-IT is its modular nature, allowing to the identification of optimized combinations of protein-stability domains, receptor-targeting domains, and inhibitory domains. To understand whether the concept of small molecule–controlled delivery of inhibitory domains can be extended to other systems, we explored proteolysis targeting chimera (PROTAC)–mediated protein control (Fig. 4A and B). To this end, the Zap70-PD1 domain was fused to the FKBP12F36V domain, to achieve control over CRASH-IT protein levels using the heterobifunctional dTAG-13 molecule that induces CRBN E3 ligase–dependent proteasomal degradation (32). Notably, FKBP12F36V-based CRASH-IT switches allowed very tight control over CD8+ T-cell function, with near absent production of IFNγ, IL2, TNFα, and cell-surface LAMP1 in the absence of drug (1.08 ± 0.20%, 0.39 ± 0.02%, 0.96 ± 0.09%, 0.82 ± 0.35%, respectively), and induction to high levels (75.43 ± 0.29%, 54.80 ± 1.81%, 80.17 ± 0.31%, 41.53 ± 1.53% at 0.5 μmol/L dTAG-13, respectively) when inhibitory tail–degradation was induced (Fig. 4C–E). Using sorted CDK4 TCR and Zap70-PD1-FKBP12F36V (EGFP) cotransduced CD8+ T cells, control over T-cell function by drug addition was demonstrated in cytotoxicity assays, whereas the activity of vector-modified T cells was unaltered (Fig. 4F and G). As compared with SMASh-based switch–expressing cells, use of the FKBP12F36V-based CRASH-IT switch substantially improved control of restoration of cytokine production in CD4 cells (Supplementary Fig. S6A–S6C) and improved control of cytotoxicity by CD8+ T cells (Fig. 4F and G; Supplementary Fig. S6D and S6E), emphasizing that the overall performance of these switch systems can be enhanced by altering their constituent modular domains.
Figure 4.
A PROTAC-based CRASH-IT system. A and B, Schematic diagram of the Zap70-PD1-FKBP12F36V fusion protein in the absence (A) or presence (B) of dTAG-13 PROTAC. Primary human T cells modified with the CDK4 TCR and either Zap70-PD1-SMASh, Zap70-PD1-FKBP12F36V, or vector control were pretreated with the indicated concentrations of the HCV NS3/4A protease inhibitors asunaprevir (C) or grazoprevir (D), or the dTAG-13 PROTAC (E). Data depict intracellular IFNγ, IL2, TNFα and cell-surface LAMP1 expression of CDK4 TCR+, EGFP-high CD8+ T cells upon coculture with NKIRTIL006 tumor cells in the continued presence of the indicated concentrations of asunaprevir, grazoprevir, or dTAG-13. Regulation of T-cell cytotoxicity with a small-molecule controlled Zap70-PD1-FKBP12F36V switch. Primary human T cells modified with the MHC class I–restricted CDK4 TCR and either Zap70-PD1-FKBP12F36V (F) or vector control (G) were sorted for CD8 and high EGFP expression, expanded by REP, and then pretreated with 0.5 μmol/L dTAG-13 or DMSO. Data depict 51Cr release from labeled NKIRTIL006 tumor cells upon coculture with sorted CD8+ CDK4 TCR+ EGFP-high T cells in the continued presence or absence of dTAG-13. E:T ratio, effector:target ratio. Data depict EGFP (H) and transgenic TCR (I) expression profiles of cells at the end of rapid expansion protocol. Error bars represent SD (n = 3). Data are representative of two independent experiments.
Intracellular staining of HA-tagged Zap70-PD1-FKBP12F36V–expressing cells revealed PROTAC-mediated degradation of fusion protein (Supplementary Fig. S7A and S7B), but a substantial HA-protein pool remained detectable at drug concentrations that induced restoration of T-cell functions in NKIRTIL006 tumor coculture experiments (Fig. 4E). Likewise, residual HA positivity was observed in experiments using SMASh-based switch–expressing cells (Fig. 2C). Collectively, these results suggest that partial degradation of switch molecules or dislocation of the switch away from ITAM receptors may contribute to restoration of T-cell functions. Like cells expressing the SMASh-based switch (Supplementary Fig. S3D), FKBP12F36V-based switch–expressing cells tolerated prior exposure to small molecule, with fully preserved control over T-cell activity after multiple prior rounds of PROTAC treatment (Supplementary Fig. S7C–S7E).
To assess the utility of CRASH-IT platform to control T cells employing endogenous TCR complexes, we modified primary human T cells with Zap70-PD1-FKBP12F36V and stimulated endogenous TCR complexes using immobilized anti-CD3 or anti-CD3/CD28. T-cell functionality was again suppressed by CRASH-IT and regained by drug addition (Supplementary Fig. S8).
When T cells were stimulated with anti-CD3/CD28 beads prior to transduction with the CRASH-IT switch and expanded in vitro in the absence of small molecule, a reduction in the fraction of EGFP+ cells emerged after 3 to 4 weeks. In parallel experiments, long-term outgrowth of T cells in the presence of small molecule resulted in T-cell expansion without outgrowth of EGFP− cells (Fig. 4H and I). Together these results indicate that strategies that guarantee stable expression of CRASH-IT switches, for instance through the use of single vector systems (33) that combine CAR or TCR and switch, or through combination of CRASH-IT with an in vivo selection system (34), may be attractive to avoid loss of switch expression. In addition, intermittent/low-dose application of small molecule might be desirable to allow long-term engraftment of switch-modified cells in vivo. Intermittent dosing would result in a situation akin to the cyclical tonic TCR signaling that T cells receive in vivo, in which low level tonic TCR signaling appears to occur in lymphoid organs but not in blood (35). In addition, the constitutive tonic signaling associated with certain CAR constructs has been linked to excessive proliferation and exhaustion (35), and it would be interesting to assess whether the negative effects of this type of tonic signaling may be reduced by intermittent drug administration.
CRASH-IT is a titratable and reversible safety switch platform that is agnostic to the nature of the activating antigen receptor, as shown by its use together with high and intermediate affinity MHC class I–restricted TCRs, MHC class II–restricted TCRs, and CARs. The flexible nature of CRASH-IT has the potential to be of value in settings in which fine control over T-cell sensitivity is desirable. Furthermore, combination of CRASH-IT with existing CARs does not require a structural redesign of CARs, and this ability to retrofit is of particular value for CAR designs that have already shown activity in clinical studies.
From a conceptual point of view, CRASH-IT switches contain three functional elements that induce proximity to the antigen receptor, provide the inhibitory signal, and offer the possibility to regulate the strength of this inhibitory signal. In the design that we have developed, these three functional elements are formed by the Zap70 SH2 domains, the PD1 tail and the SMASh tag or FKBP12F36V domain, respectively. In future work, it will be of interest to investigate whether this blueprint can achieve optimal control in other cells, such as natural killer cells, and whether it is possible to achieve inhibition of other activating immune receptors. In addition, it will be of interest to explore alternative inhibitory domains from receptors containing immunoreceptor tyrosine-based inhibitory motifs (ITIM) or immunoreceptor tyrosine-based switch motifs (ITSM) as a means to vary the level of T-cell suppression, or to potentially direct such suppression to specific T-cell output signals. Akin to the development of novel generation CAR receptors, we envision that such optimization of functional modules will increase the clinical utility of the CRASH-IT system. In this regard, development of systems that allow control with a clinically improved small-molecule ligands (16) will also be of interest.
Authors' Disclosures
A.C. Sahillioglu reports a patent for WO2021080427A1 pending. T.N. Schumacher reports grants from Oncode Institute during the conduct of the study; personal fees from Asher Biotherapeutics, Allogene Therapeutics, Merus, Adaptive Biotechnologies, Neogene Therapeutics, and Third Rock Ventures and grants from Merck KGaA outside the submitted work; and a patent for WO2021080427A1, Chimeric Polypeptide for Regulating Immune Cells, pending. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
The authors thank M.H. Heemskerk (LUMC, Leiden, the Netherlands) for providing class II CMV TCR sequences and the CBH-5477 cell line, R.N. Ozdeslik (UMC Utrecht, Utrecht, the Netherlands) for illustrations, and M. Marsman for valuable suggestions.
This research was supported by the Oncode Institute.
Footnotes
Note: Supplementary data for this article are available at Cancer Immunology Research Online (http://cancerimmunolres.aacrjournals.org/).
Current address for R. Gomez: Neogene Therapeutics, Amsterdam, the Netherlands.
Cancer Immunol Res 2021;9:999–1007
Authors' Contributions
A.C. Sahillioglu: Conceptualization, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. M. Toebes: Investigation. G. Apriamashvili: Investigation. R. Gomez: Investigation. T.N. Schumacher: Conceptualization, supervision, writing–original draft, writing–review and editing.
References
- 1. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018;359:1361–5. [DOI] [PubMed] [Google Scholar]
- 3. Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol 2008;21:205–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer 2018;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Agostino M, Raje N. Anti-BCMA CAR T-cell therapy in multiple myeloma: can we do better? Leukemia 2020;34:21–34. [DOI] [PubMed] [Google Scholar]
- 6. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016;3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013;36:133–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front Immunol 2020;11:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol 2014;5:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med 2019;11:eaau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 2015;350:aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A 2016;113:E450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loureiro LR, Feldmann A, Bergmann R, Koristka S, Berndt N, Arndt C, et al. Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells. Blood Cancer J 2018;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Juillerat A, Tkach D, Busser BW, Temburni S, Valton J, Duclert A, et al. Modulation of chimeric antigen receptor surface expression by a small molecule switch. BMC Biotechnol 2019;19:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SM, Kang CH, Choi SU, Kim Y, Hwang JY, Jeong HG, et al. A chemical switch system to modulate chimeric antigen receptor T cell activity through proteolysis-targeting chimaera technology. ACS Synth Biol 2020;9:987–92. [DOI] [PubMed] [Google Scholar]
- 16. Jan M, Scarfo I, Larson RC, Walker A, Schmidts A, Guirguis AA, et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci Transl Med 2021;13:eabb6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med 2013;5:215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engels B, Cam H, Schuler T, Indraccolo S, Gladow M, Baum C, et al. Retroviral vectors for high-level transgene expression in T lymphocytes. Hum Gene Ther 2003;14:1155–68. [DOI] [PubMed] [Google Scholar]
- 19. Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 2009;6:343–5. [DOI] [PubMed] [Google Scholar]
- 20. Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13:5426–35. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011;118:1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stronen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 2016;352:1337–41. [DOI] [PubMed] [Google Scholar]
- 23. Linnemann C, Heemskerk B, Kvistborg P, Kluin RJ, Bolotin DA, Chen X, et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 2013;19:1534–41. [DOI] [PubMed] [Google Scholar]
- 24. van Loenen MM, Hagedoorn RS, de Boer R, Falkenburg JH, Heemskerk MH. Extracellular domains of CD8alpha and CD8ss subunits are sufficient for HLA class I restricted helper functions of TCR-engineered CD4(+) T cells. PLoS One 2013;8:e65212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology 2012;1:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol 2006;177:6548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young L, Sung J, Stacey G, Masters JR. Detection of Mycoplasma in cell cultures. Nat Protoc 2010;5:929–34. [DOI] [PubMed] [Google Scholar]
- 28. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–7. [DOI] [PubMed] [Google Scholar]
- 29. Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012;209:1201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qian D, Mollenauer MN, Weiss A. Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med 1996;183:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chung HK, Jacobs CL, Huo Y, Yang J, Krumm SA, Plemper RK, et al. Tunable and reversible drug control of protein production via a self-excising degron. Nat Chem Biol 2015;11:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nabet B, Roberts JM, Buckley DL, Paulk J, Dastjerdi S, Yang A, et al. The dTAG system for immediate and target-specific protein degradation. Nat Chem Biol 2018;14:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol 2005;23:108–16. [DOI] [PubMed] [Google Scholar]
- 34. Jonnalagadda M, Brown CE, Chang WC, Ostberg JR, Forman SJ, Jensen MC. Engineering human T cells for resistance to methotrexate and mycophenolate mofetil as an in vivo cell selection strategy. PLoS One 2013;8:e65519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ajina A, Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol Cancer Ther 2018;17:1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.