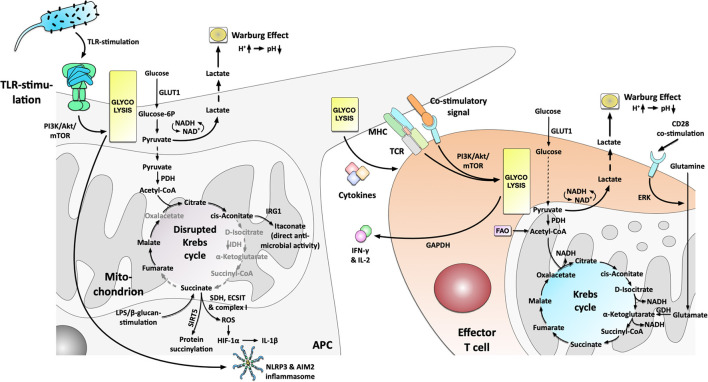
Figure 2.
Metabolic phenotype and the connection to immune cell effector function in activated Antigen Presenting Cells and effector T cells. Antigen Presenting Cells (APCs) activated by e.g., TLR-stimulation switch to a predominant production of energy via glycolysis that drives cytokine production and results in the production and excretion of lactate (Warburg Effect). The switch toward glycolysis is driven by the PI3K/Akt/mTOR-axis. The disrupted Krebs cycle resulting from an undersupply of pyruvate in the mitochondrion is used to (I) generate important immune effector molecules (e.g., itaconate, ROS), (II) fuel post-translational protein modification (e.g., succinylation or acetylation), and (III) free up the mitochondria from energy production in order to promote inflammatory responses via an SDH-, ECSIT- and complex I-dependent production of ROS which in turn drive HIF-1α- and inflammasome-dependent IL-1β production. Activated T cells PI3K/Akt/mTOR-dependently increase their glycolysis but also retain metabolic flexibility with the ability to fuel the mitochondrion with glutamine. Increased glutamine uptake and metabolism is regulated by CD28-dependent ERK-signaling. Moreover, the glycolytic enzyme GAPDH regulated IFN-γ secretion by interacting with IFN-γ mRNA. For more information see text. TLR, “Toll”-like receptor; GLUT1, glucose transporter 1; PDH, pyruvate dehydrogenase; IRG-1, immune-responsive gene 1; IDH, isocitrate dehydrogenase; SDH, succinate dehydrogenase; ROS, reactive oxygen species; HIF-1α, hypoxia inducible factor 1 alpha; SIRT5, Sirtuin 5; ECSIT, evolutionarily conserved signaling intermediate in Toll pathways; MHC, major histocompatibility complex; TCR, T cell receptor; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GDH, glutamate dehydrogenase; ERK, extracellular regulated kinase; FAO, fatty acid oxidase.