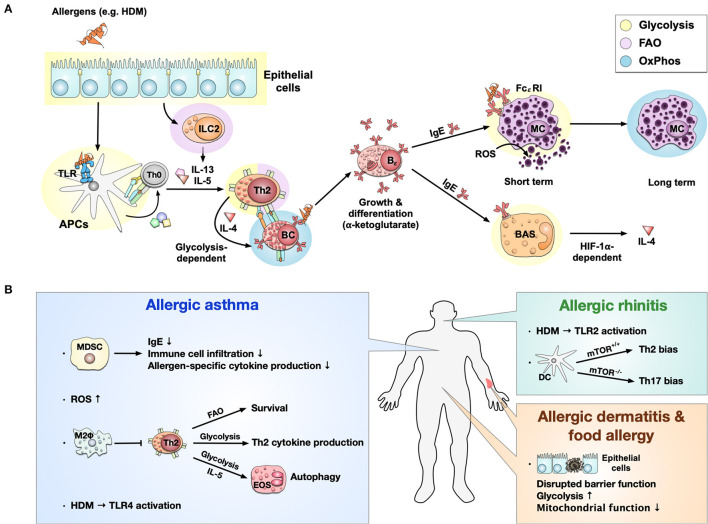
Figure 3.
Immune metabolism in allergies. Immune metabolism in allergic sensitization and pathology (A). Allergens, e.g., derived from house dust mites (HDM), activate epithelial cells and induce a metabolic shift toward glycolysis. Epithelial cells further activate ILC2s and induce FAO-dependent production of the Th2 cytokines IL-5 and IL-13. Some allergens can also bind to TLRs, causing a switch from OxPhos to glycolysis in APCs. Activated ILC2s and APCs further induce the differentiation of naïve T cells into Th2 cells which produce IL-4 in a glycolysis-dependent manner. The activation and differentiation of B cells into IgE-producing plasma cells after IL-4 stimulation is dependent on the Krebs cycle-derived metabolite α-ketoglutarate. The produced IgE binds to the high-affinity FCεRI on mast cells, where cross-linking of IgE leads to a short-term glycolytic phenotype. In contrast, long-term mast cell activation is dependent on OxPhos. Additionally, high concentrations of ROS can directly activate mast cells to release histamine and serotonin. On the other hand, IgE can also bind to basophils, leading to a HIF-1α-dependent activation of glycolysis and subsequent IL-4 production. Immune metabolic changes observed in allergic asthma, rhinitis, dermatitis, and food allergy (B). In allergic asthma, MDSCs have been shown to inhibit allergic asthma by reducing IgE production, immune cell infiltration, and allergen-specific cytokine production. At the same time, high levels of reactive oxygen species (ROS) were found. OxPhos-based anti-inflammatory M2 macrophages may locally inhibit Th2 responses in allergic asthma. Furthermore, for Th2 cells, FAO-dependency could be identified to be essential for survival, while glycolysis was essential for Th2 cytokine production. IL-5 can activate eosinophils, shift their metabolism toward a glycolytic phenotype, and affect their effector functions like autophagy in asthma. Allergic asthma induced by HDM was shown to be TLR4-dependent; while HDM-induced allergic rhinitis was driven by TLR2 activation. In allergic rhinitis, mTOR-deficiency in CD11b+ DCs induced Th17- instead of Th2-biased immune responses. While little is known about immune metabolism in allergic dermatitis and food allergy, they are both connected to a disrupted barrier function of epithelial cells characterized by a predominantly glycolytic phenotype and mitochondrial dysfunction. TLR, “Toll”-like receptor; APC, antigen presenting cell; MC, mast cell; BAS, basophil; ROS, reactive oxygen species; HIF-1α, hypoxia inducible factor 1 alpha; MHC, major histocompatibility complex; mTOR, mammalian target of rapamycin; FAO, fatty acid oxidation; HDM, house dust mite; IgE, Immunoglobulin E; Bϵ, IgE-producing B cell; BC, B cell; TC, T cell; OxPhos, oxidative phosphorylation; MDSC, Myeloid-derived suppressor cell; FcεRI, fragment crystallizable region epsilon receptor I; M2Φ, M2-macrophages; EOS, eosinophils.