Abstract
Background
Patent ductus arteriosus (PDA) is associated with significant morbidity and mortality in preterm infants. Cyclooxygenase inhibitors (COX‐I) may prevent PDA‐related complications. Controversy exists on which COX‐I drug is the most effective and has the best safety profile in preterm infants.
Objectives
To compare the effectiveness and safety of prophylactic COX‐I drugs and 'no COXI prophylaxis' in preterm infants using a Bayesian network meta‐analysis (NMA).
Search methods
Searches of Cochrane CENTRAL via Wiley, OVID MEDLINE and Embase via Elsevier were conducted on 9 December 2021. We conducted independent searches of clinical trial registries and conference abstracts; and scanned the reference lists of included trials and related systematic reviews.
Selection criteria
We included randomised controlled trials (RCTs) that enrolled preterm or low birth weight infants within the first 72 hours of birth without a prior clinical or echocardiographic diagnosis of PDA and compared prophylactic administration of indomethacin or ibuprofen or acetaminophen versus each other, placebo or no treatment.
Data collection and analysis
We used the standard methods of Cochrane Neonatal. We used the GRADE NMA approach to assess the certainty of evidence derived from the NMA for the following outcomes: severe intraventricular haemorrhage (IVH), mortality, surgical or interventional PDA closure, necrotizing enterocolitis (NEC), gastrointestinal perforation, chronic lung disease (CLD) and cerebral palsy (CP).
Main results
We included 28 RCTs (3999 preterm infants). Nineteen RCTs (n = 2877) compared prophylactic indomethacin versus placebo/no treatment, 7 RCTs (n = 914) compared prophylactic ibuprofen versus placebo/no treatment and 2 RCTs (n = 208) compared prophylactic acetaminophen versus placebo/no treatment. Nine RCTs were judged to have high risk of bias in one or more domains.We identified two ongoing trials on prophylactic acetaminophen.
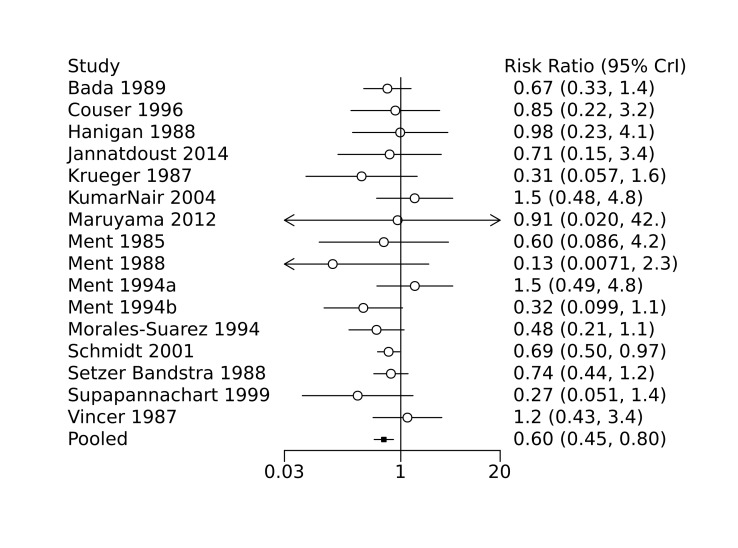
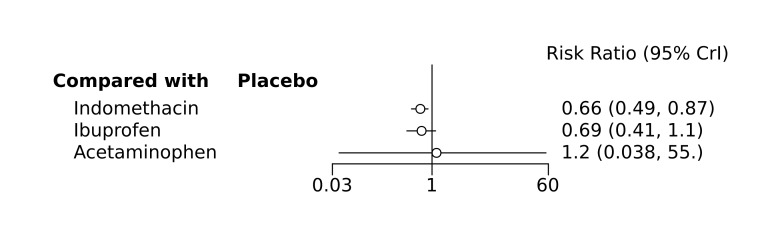
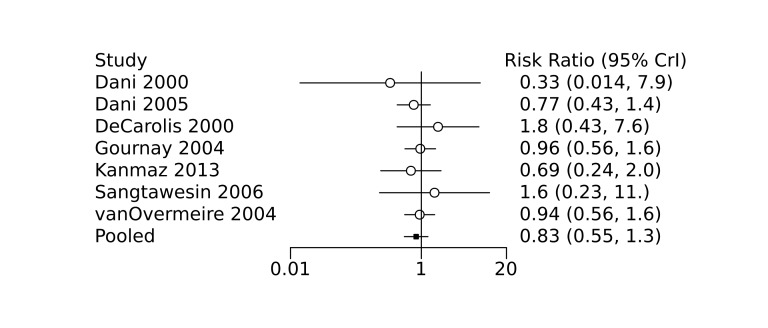
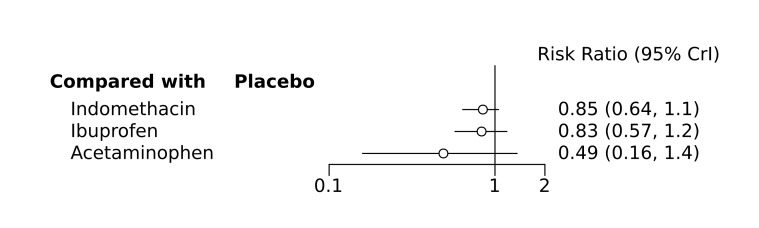
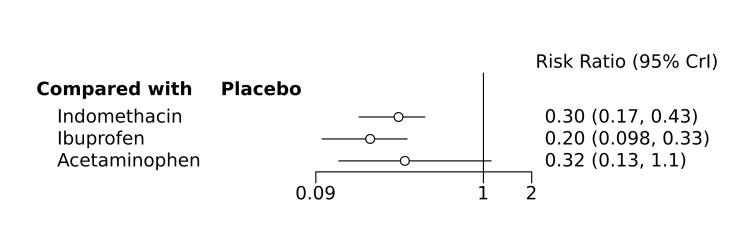
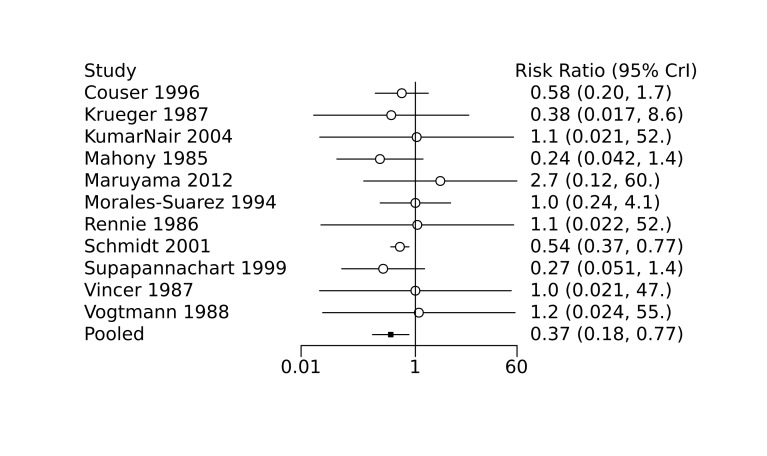
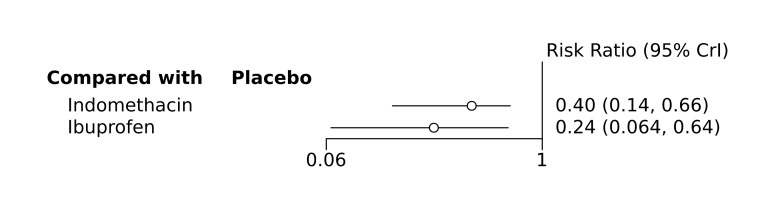
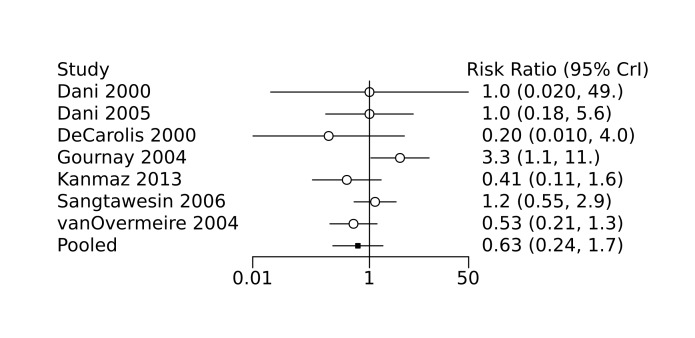
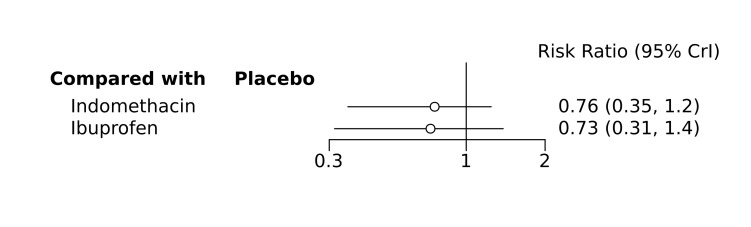
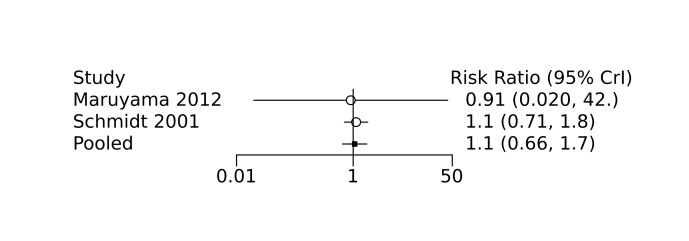
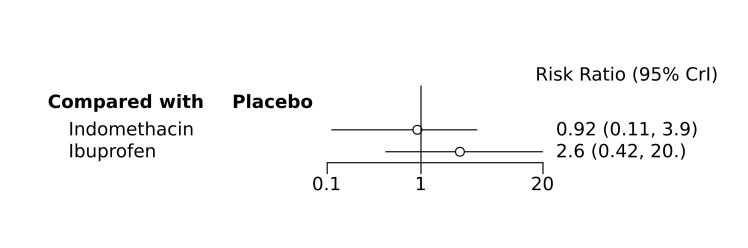
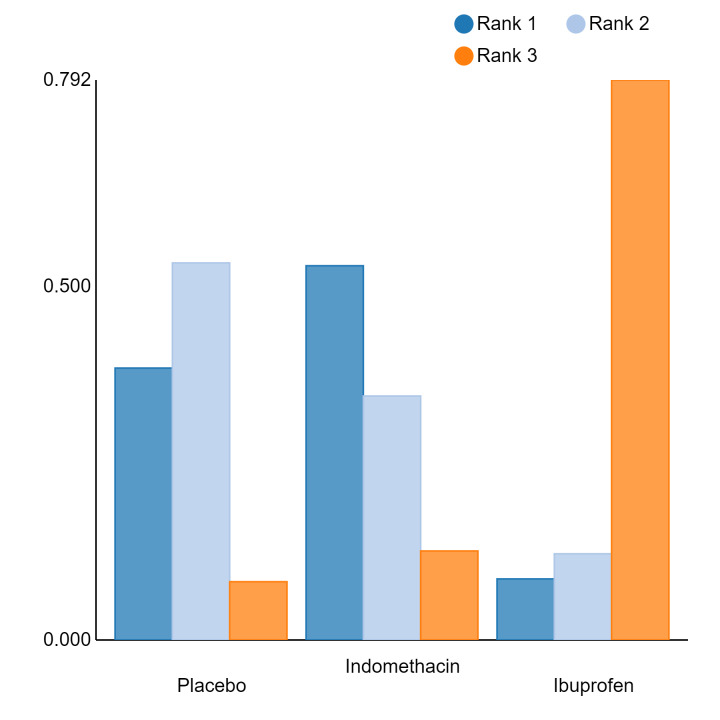
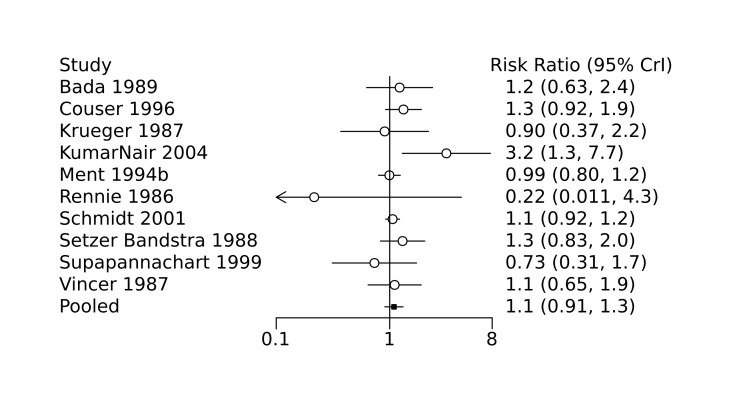
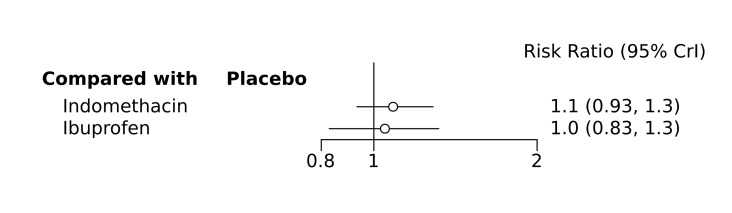
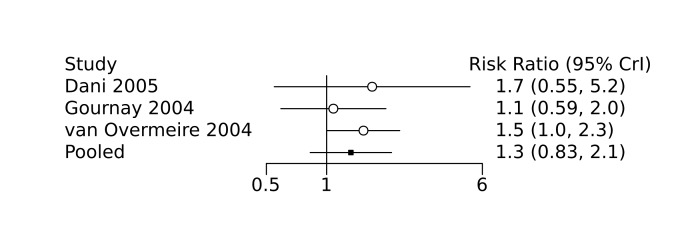
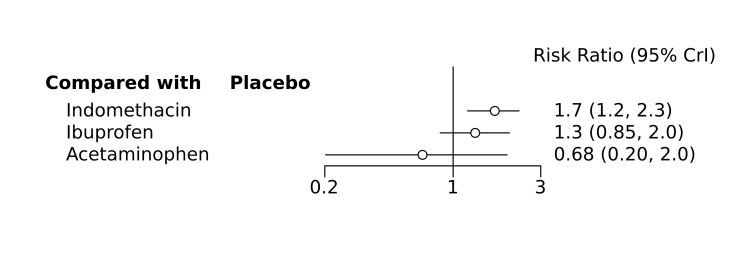
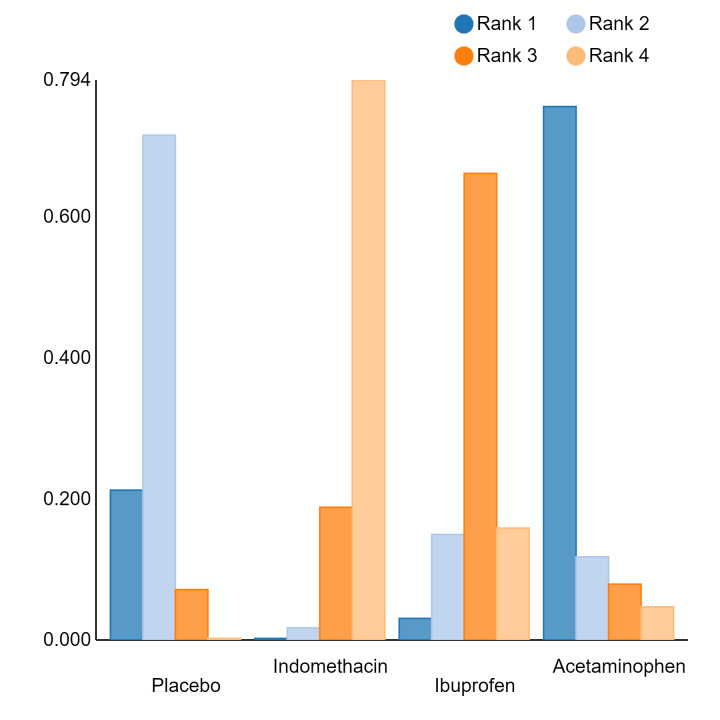
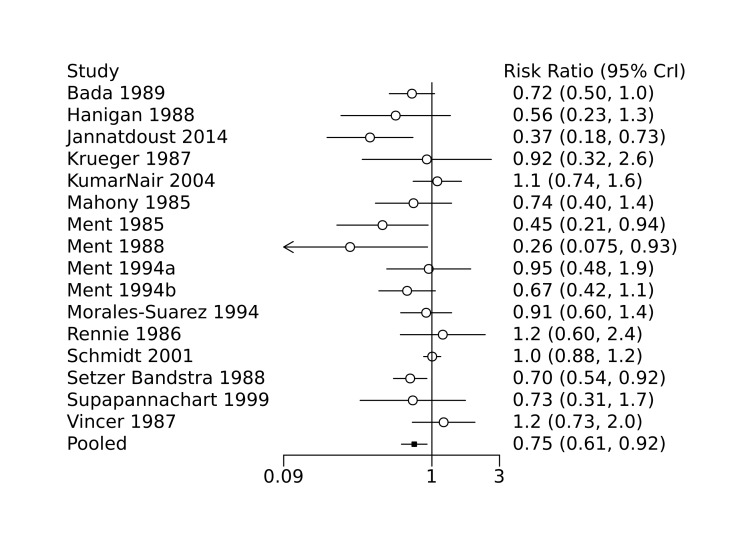
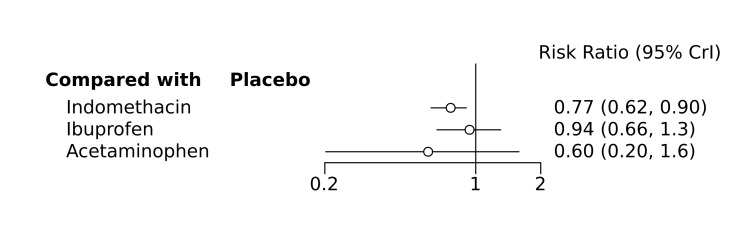
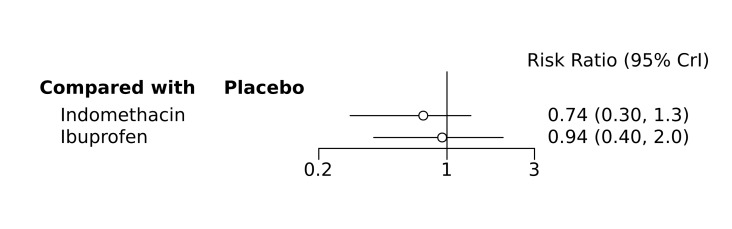
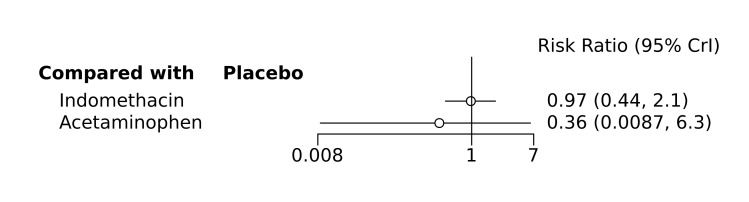
Bayesian random‐effects NMA demonstrated that prophylactic indomethacin probably led to a small reduction in severe IVH (network RR 0.66, 95% Credible Intervals [CrI] 0.49 to 0.87; absolute risk difference [ARD] 43 fewer [95% CrI, 65 fewer to 16 fewer] per 1000; median rank 2, 95% CrI 1‐3; moderate‐certainty), a moderate reduction in mortality (network RR 0.85, 95% CrI 0.64 to 1.1; ARD 24 fewer [95% CrI, 58 fewer to 16 more] per 1000; median rank 2, 95% CrI 1‐4; moderate‐certainty) and surgical PDA closure (network RR 0.40, 95% CrI 0.14 to 0.66; ARD 52 fewer [95% CrI, 75 fewer to 30 fewer] per 1000; median rank 2, 95% CrI 1‐2; moderate‐certainty) compared to placebo. Prophylactic indomethacin resulted in trivial difference in NEC (network RR 0.76, 95% CrI 0.35 to 1.2; ARD 16 fewer [95% CrI, 42 fewer to 13 more] per 1000; median rank 2, 95% CrI 1‐3; high‐certainty), gastrointestinal perforation (network RR 0.92, 95% CrI 0.11 to 3.9; ARD 4 fewer [95% CrI, 42 fewer to 137 more] per 1000; median rank 1, 95% CrI 1‐3; moderate‐certainty) or CP (network RR 0.97, 95% CrI 0.44 to 2.1; ARD 3 fewer [95% CrI, 62 fewer to 121 more] per 1000; median rank 2, 95% CrI 1‐3; low‐certainty) and may result in a small increase in CLD (network RR 1.10, 95% CrI 0.93 to 1.3; ARD 36 more [95% CrI, 25 fewer to 108 more] per 1000; median rank 3, 95% CrI 1‐3; low‐certainty).
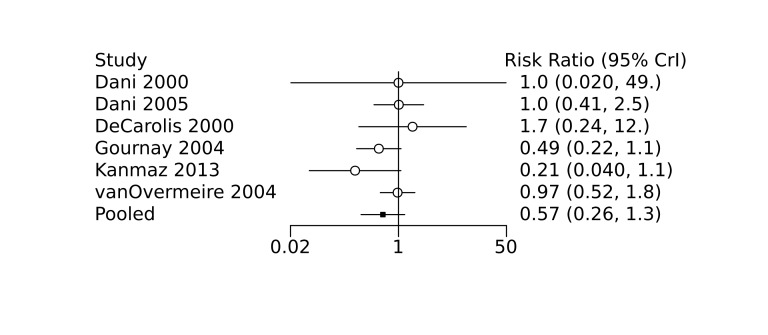
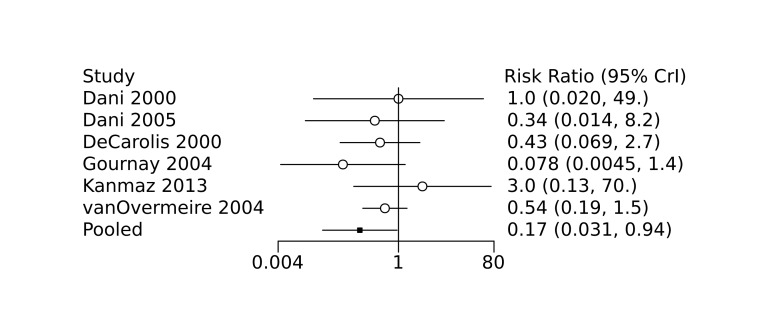
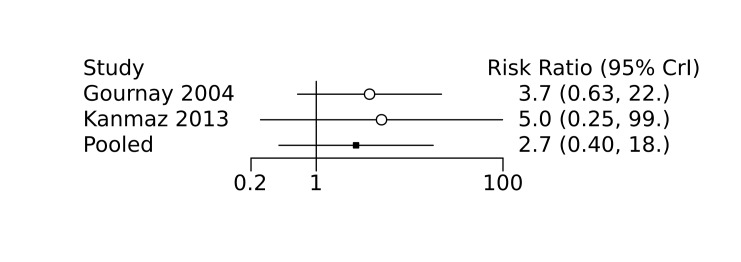
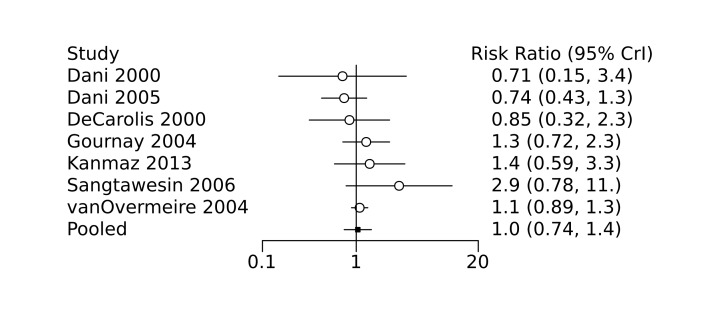
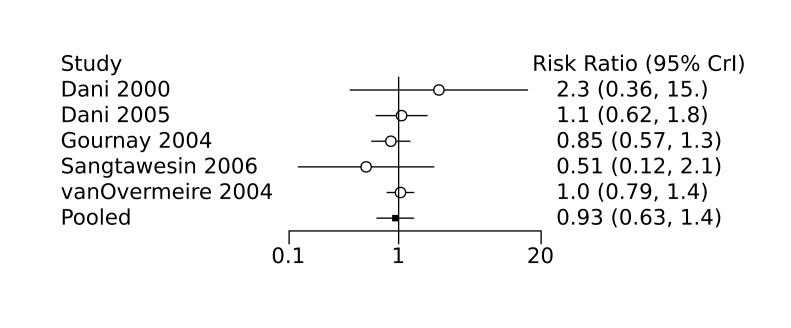
Prophylactic ibuprofen probably led to a small reduction in severe IVH (network RR 0.69, 95% CrI 0.41 to 1.14; ARD 39 fewer [95% CrI, 75 fewer to 18 more] per 1000; median rank 2, 95% CrI 1‐4; moderate‐certainty) and moderate reduction in surgical PDA closure (network RR 0.24, 95% CrI 0.06 to 0.64; ARD 66 fewer [95% CrI, from 82 fewer to 31 fewer] per 1000; median rank 1, 95% CrI 1‐2; moderate‐certainty) compared to placebo. Prophylactic ibuprofen may result in moderate reduction in mortality (network RR 0.83, 95% CrI 0.57 to 1.2; ARD 27 fewer [95% CrI, from 69 fewer to 32 more] per 1000; median rank 2, 95% CrI 1‐4; low‐certainty) and leads to trivial difference in NEC (network RR 0.73, 95% CrI 0.31 to 1.4; ARD 18 fewer [95% CrI, from 45 fewer to 26 more] per 1000; median rank 1, 95% CrI 1‐3; high‐certainty), or CLD (network RR 1.00, 95% CrI 0.83 to 1.3; ARD 0 fewer [95% CrI, from 61 fewer to 108 more] per 1000; median rank 2, 95% CrI 1‐3; low‐certainty). The evidence is very uncertain on effect of ibuprofen on gastrointestinal perforation (network RR 2.6, 95% CrI 0.42 to 20.0; ARD 76 more [95% CrI, from 27 fewer to 897 more] per 1000; median rank 3, 95% CrI 1‐3; very low‐certainty).
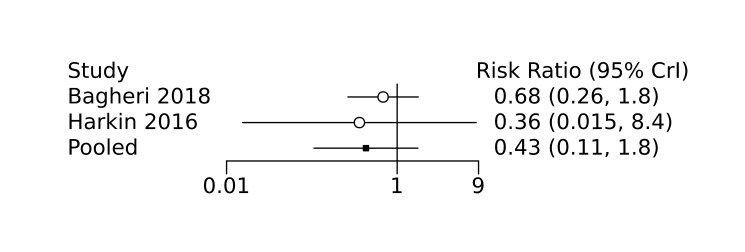
The evidence is very uncertain on the effect of prophylactic acetaminophen on severe IVH (network RR 1.17, 95% CrI 0.04 to 55.2; ARD 22 more [95% CrI, from 122 fewer to 1000 more] per 1000; median rank 4, 95% CrI 1‐4; very low‐certainty), mortality (network RR 0.49, 95% CrI 0.16 to 1.4; ARD 82 fewer [95% CrI, from 135 fewer to 64 more] per 1000; median rank 1, 95% CrI 1‐4; very low‐certainty), or CP (network RR 0.36, 95% CrI 0.01 to 6.3; ARD 70 fewer [95% CrI, from 109 fewer to 583 more] per 1000; median rank 1, 95% CrI 1‐3; very low‐certainty).
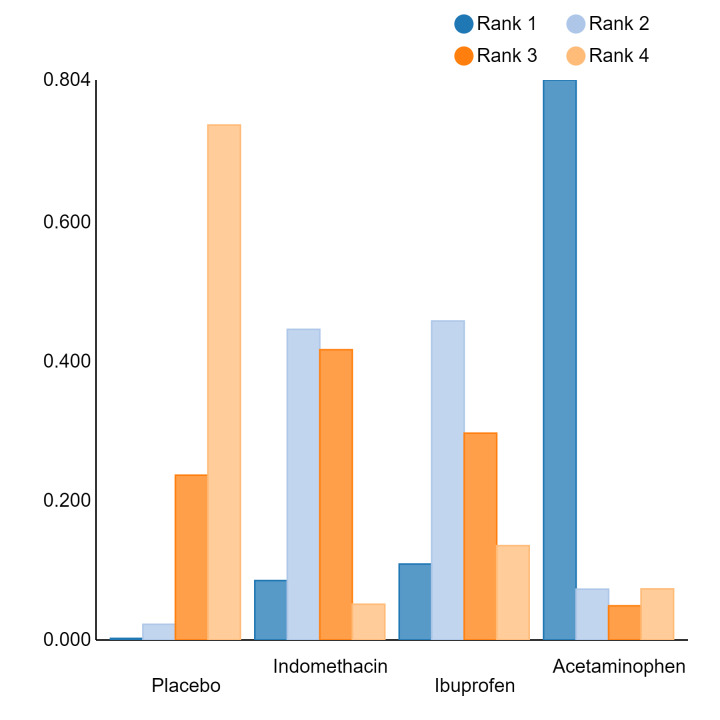
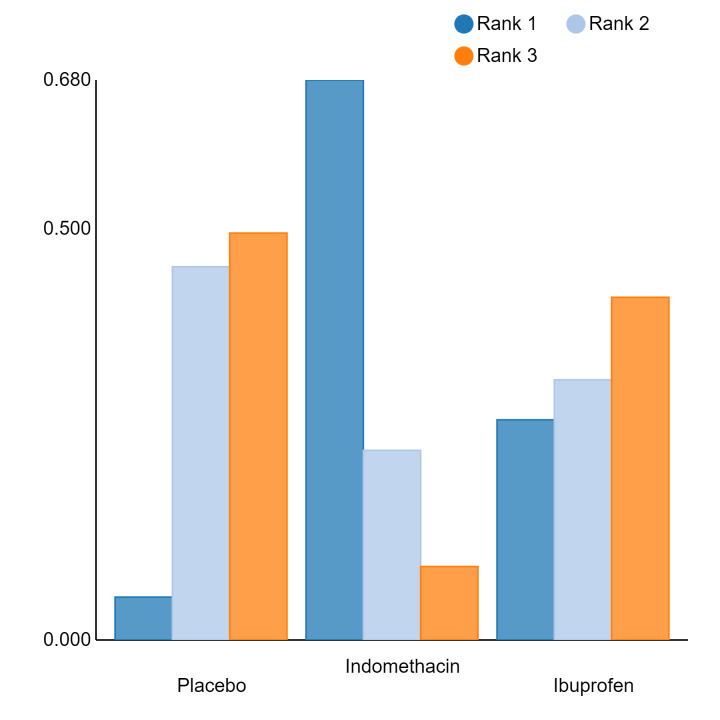
In summary, based on ranking statistics, both indomethacin and ibuprofen were equally effective (median ranks 2 respectively) in reducing severe IVH and mortality. Ibuprofen (median rank 1) was more effective than indomethacin in reducing surgical PDA ligation (median rank 2). However, no statistically‐significant differences were observed between the COX‐I drugs for any of the relevant outcomes.
Authors' conclusions
Prophylactic indomethacin probably results in a small reduction in severe IVH and moderate reduction in mortality and surgical PDA closure (moderate‐certainty), may result in a small increase in CLD (low‐certainty) and results in trivial differences in NEC (high‐certainty), gastrointestinal perforation (moderate‐certainty) and cerebral palsy (low‐certainty). Prophylactic ibuprofen probably results in a small reduction in severe IVH and moderate reduction in surgical PDA closure (moderate‐certainty), may result in a moderate reduction in mortality (low‐certainty) and trivial differences in CLD (low‐certainty) and NEC (high‐certainty). The evidence is very uncertain about the effect of acetaminophen on any of the clinically‐relevant outcomes.
Plain language summary
Prophylactic cyclo‐oxygenase inhibitor drugs to prevent morbidity and mortality in preterm infants
Review question
Among the available cyclo‐oxygenase inhibitor (COX‐I) drugs (indomethacin, ibuprofen, acetaminophen), which one is the safest and most effective in preventing death and poor outcomes in preterm infants when given prophylactically without the prior knowledge of the presence of a patent ductus arteriosus (PDA) within the first 72 hours after birth?
Background
A PDA is a common complication in preterm or low‐birth weight infants. PDA is an open vascular channel between the lungs and the heart which usually closes shortly after birth. In preterm infants, the PDA frequently remains open and may contribute to life‐threatening complications. COX‐I drugs such as indomethacin, ibuprofen and acetaminophen may prevent a PDA and related poor outcomes. Controversy exists on which of the three COX‐I drugs, if any, improves clinical outcomes in preterm infants.
Study characteristics
We searched scientific databases for randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) in preterm babies (born at less than 37 weeks into pregnancy) or low‐birthweight (weighing less than 2500 grams) infants where COX‐I drugs were given without the prior knowledge of the presence of a PDA, within the first 72 hours after birth. The included studies compared administration of indomethacin or ibuprofen or acetaminophen versus each other, placebo or no treatment.
Key results
This review of 28 clinical trials (3999 preterm infants) found that prophylactic indomethacin probably results in a small reduction in severe brain bleeding, a moderate reduction in death and need for PDA surgery, and may result in a small increase in chronic lung disease. Prophylactic indomethacin likely results in trivial differences in necrotizing enterocolitis, gastrointestinal perforation and cerebral palsy. Prophylactic ibuprofen probably results in a small reduction in severe brain bleeding and a moderate reduction in need for PDA surgery. Prophylactic ibuprofen may result in a moderate reduction in death and trivial differences in chronic lung disease and necrotizing enterocolitis. The evidence is very uncertain about the effect of acetaminophen on any of the clinically relevant outcomes.There are currently two ongoing trials on prophylactic use of acetaminophen.
Certainty of the evidence
According to GRADE (a method to score the certainty of the trials supporting each outcome), the certainty of the evidence varied from very low to high but was moderate for the most important outcomes of severe brain bleeding and death.
How up to date is the search evidence
The search is up to date as of 9 December 2021.
Summary of findings
Summary of findings 1. Summary of findings.
| Outcome | Effects and confidence in the effect estimates | Comments** | |||||
| Indomethacin | Ibuprofen | Acetaminophen | |||||
| Severe Intraventricular Haemorrhage | |||||||
|
Placebo comparator 127 per 1000 (12.7%) |
Network RR 0.66 (0.49, 0.87) |
Network absolute risk difference* 43 fewer per 1000 (from 65 fewer to 16 fewer) |
Network RR 0.69 (0.41, 1.14) |
Network absolute risk difference 39 fewer per 1000 (from 75 fewer to 18 more) |
Network RR 1.17 (0.04, 55.2) |
Network absolute risk difference 22 more per 1000 (from 122 fewer to 1000 more) |
· Prophylactic indomethacin probably results in a small reduction in severe IVH · Prophylactic ibuprofen probably results in a small reduction in severe IVH · The evidence is very uncertain about the effect of prophylactic acetaminophen on severe IVH |
|
Moderate ⊕⊕⊕◯ Confidence in estimate due to imprecision1 |
Moderate ⊕⊕⊕◯ Confidence in estimate due to imprecision2 |
Very Low ⊕◯◯◯ Confidence in estimate due to imprecision3 |
|||||
|
Rank [Median (95% CrIs)] 3 (2‐4) |
Rank 2 (1‐3) |
Rank 2 (1‐4) |
Rank 4 (1‐4) |
||||
| Based on 2629 infants (16 RCTs) | Based on 863 infants (6 RCTs) | Based on 48 infants (1 RCT) | |||||
| Mortality | |||||||
|
Placebo comparator 161 per 1000 (16.1%) |
Network RR 0.85 (0.64 to 1.1) |
Network absolute risk difference 24 fewer per 1000 (from 58 fewer to 16 more) |
Network RR 0.83 (0.57 to 1.2) |
Network absolute risk difference 27 fewer per 1000 (from 69 fewer to 32 more) |
Network RR 0.49 (0.16 to 1.4) |
Network absolute risk difference 82 fewer per 1000 (from 135 fewer to 64 more) |
· Prophylactic indomethacin probably results in a moderate reduction in mortality · Prophylactic ibuprofen may result in a moderate reduction in mortality · The evidence is very uncertain about the effect of prophylactic acetaminophen on mortality |
|
Moderate ⊕⊕⊕◯ Confidence in estimate due to imprecision4 |
Low ⊕⊕◯◯ Confidence in estimate due to imprecision5 |
Very Low ⊕◯◯◯ Confidence in estimate due to risk of bias and imprecision6 |
|||||
|
Rank [Median (95% CrIs)] 4 (3‐4) |
Rank 2 (1‐4) |
Rank 2 (1‐4) |
Rank 1 (1‐4) |
||||
| Based on 2877 infants (19 RCTs) |
Based on 914 infants (7 RCTs) | Based on 208 infants (2 RCTs) | |||||
| Surgical PDA closure | |||||||
|
Placebo comparator 87 per 1000 (8.7%) |
Network RR 0.40 (0.14 to 0.66) |
Network absolute risk difference 52 fewer per 1000 (from 75 fewer to 30 fewer) |
Network RR 0.24 (0.06 to 0.64) |
Network absolute risk difference 66 fewer per 1000 (from 82 fewer to 31 fewer) |
_______ | _______ | · Prophylactic indomethacin probably results in a moderate reduction in need for surgical PDA closure · Prophylactic ibuprofen probably results in a moderate reduction in need for surgical PDA closure · There is no evidence on the effect of prophylactic acetaminophen on need for surgical PDA closure |
|
Moderate ⊕⊕⊕◯ Confidence in estimate due to imprecision7 |
Moderate ⊕⊕⊕◯ Confidence in estimate due to imprecision8 |
_______ | |||||
|
Rank [Median (95% CrIs)] 3 (3‐3) |
Rank 2 (1‐2) |
Rank 1 (1‐2) |
_______ | ||||
| Based on 1800 infants (11 RCTs) | Based on 873 infants (6 RCTs) | _______ | |||||
| Necrotizing Enterocolitis | |||||||
|
Placebo comparator 65 per 1000 (6.5%) |
Network RR 0.76 (0.35 to 1.2) |
Network absolute risk difference 16 fewer per 1000 (from 42 fewer to 13 more) |
Network RR 0.73 (0.31 to 1.4) |
Network absolute risk difference 18 fewer per 1000 (from 45 fewer to 26 more) |
_______ | _______ | · Prophylactic indomethacin results in trivial difference in NEC · Prophylactic ibuprofen results in trivial difference in NEC · There is no evidence on the effect of prophylactic acetaminophen on NEC |
|
High ⊕⊕⊕⊕ Confidence in estimate |
High ⊕⊕⊕⊕ Confidence in estimate |
_______ | |||||
|
Rank [Median (95% CrIs)] 3 (3‐3) |
Rank 2 (1‐3) |
Rank 1 (1‐3) |
_______ | ||||
| Based on 2543 infants (14 RCTs) | Based on 905 infants (7 RCTs) |
_______ | |||||
| Gastrointestinal perforation | |||||||
|
Placebo comparator 47 per 1000 (4.7%) |
Network RR 0.92 (0.11 to 3.9) |
Network absolute risk difference 4 fewer per 1000 (from 42 fewer to 137 more) |
Network RR 2.6 (0.42 to 20.0) |
Network absolute risk difference 76 more per 1000 (from 27 fewer to 897 more) |
_______ | _______ | · Prophylactic indomethacin probably results in trivial difference in gastrointestinal perforation · The evidence is very uncertain about the effect of prophylactic ibuprofen on gastrointestinal perforation · There is no evidence on the effect of prophylactic acetaminophen on gastrointestinal perforation |
|
Moderate ⊕⊕⊕◯ Confidence in estimate due to imprecision9 |
Very Low ⊕◯◯◯ Confidence in estimate due to imprecision10 |
_______ | |||||
|
Rank [Median (95% CrIs)] 2 (1‐3) |
Rank 1 (1‐3) |
Rank 3 (1‐3) |
_______ | ||||
| Based on 1221 infants (2 RCTs) | Based on 177 infants (2 RCTs) | _______ | |||||
| Chronic Lung Disease | |||||||
|
Placebo comparator 359 per 1000 (35.9%) |
Network RR 1.10 (0.93 to 1.3) |
Network absolute risk difference 36 more per 1000 (from 25 fewer to 108 more) |
Network RR 1.00 (0.83 to 1.3) |
Network absolute risk difference 0 fewer per 1000 (from 61 fewer to 108 more) |
_______ | _______ | · Prophylactic indomethacin may result in a small increase in chronic lung disease · Prophylactic ibuprofen may result in trivial difference in chronic lung disease · There is no evidence on the effect of prophylactic acetaminophen on chronic lung disease |
|
Low ⊕⊕◯◯ Confidence in estimate due to inconsistency and imprecision11 |
Low ⊕⊕◯◯ Confidence in estimate due to imprecision12 |
_______ | |||||
|
Rank [Median (95% CrIs)] 1 (1‐3) |
Rank 3 (1‐3) |
Rank 2 (1‐3) |
_______ | ||||
| Based on 2106 infants (10 RCTs) | Based on 904 infants (7 RCTs) |
_______ | |||||
| Cerebral Palsy | |||||||
|
Placebo comparator 110 per 1000 (11%) |
Network RR 0.97 (0.44 to 2.1) |
Network absolute risk difference 3 fewer per 1000 (from 62 fewer to 121 more) |
_______ | _______ |
Network RR 0.36 (0.01 to 6.3) |
Network absolute risk difference 70 fewer per 1000 (from 109 fewer to 583 more) |
· Prophylactic indomethacin may result in trivial difference in cerebral palsy · There is no evidence on the effect of prophylactic ibuprofen on cerebral palsy · The evidence is very uncertain about the effect of prophylactic acetaminophen on cerebral palsy |
|
Low ⊕⊕◯◯ Confidence in estimate due to imprecision13 |
_______ |
Very Low ⊕◯◯◯ Confidence in estimate due to imprecision14 |
|||||
|
Rank [Median (95% CrIs)] 2 (1‐3) |
Rank 2 (1‐3) |
_______ |
Rank 1 (1‐3) |
||||
| Based on 1367 infants (4 RCTs) | _______ | Based on 35 infants (1 RCT) | |||||
1. In the direct comparison, the credible intervals include moderate benefit (73 fewer per 1000) to small benefit (27 fewer per 1000). Therefore, the certainty of evidence was rated down by one level for imprecision. No further change was made based on the network estimates
2. In the direct comparison, the credible intervals include moderate benefit (82 fewer per 1000) to small harm (33 more per 1000). Therefore, the certainty of evidence was rated down by one level for imprecision. No further change was made based on the network estimates
3. 95% CrIs include appreciable benefit and very large harm. In the direct comparison, the certainty of evidence was rated down by one‐level for serious imprecision. Based on the network estimates, the certainty was rated down by two more levels due to very serious imprecision (implausible effect sizes) in the network estimates
4. In the direct comparison, the credible intervals include moderate benefit (61 fewer per 1000) to small harm (17 more per 1000). Therefore, the certainty of evidence was rated down by one level for imprecision. No further change was made based on the network estimates
5. In the direct comparison, the credible intervals include appreciable benefit (72 fewer per 1000) and harm (48 more per 1000). Therefore, the certainty of evidence was rated down by two levels for very serious imprecision. No further change was made based on the network estimates.
6. In the direct comparison, the certainty of evidence was rated down due to substantial risk of bias in the included studies; the certainty was further rated down two levels for very serious imprecision as the credible intervals include appreciable benefit (85 fewer per 1000) and harm (76 more per 1000). Therefore, the overall certainty of evidence for the direct estimate was rated as very low. No further change was made based on the network estimates.
7. In the direct comparison, the credible intervals include moderate benefit (88 fewer per 1000) to small benefit (25 fewer per 1000). Therefore, the certainty of evidence was rated down by one level for imprecision. No further change was made based on the network estimates
8. The certainty of evidence for the direct comparison was high. However, the 95% credible intervals in the network estimates include appreciable benefit (82 fewer) to small benefit (31 fewer). Hence, the certainty of evidence was rated down by one level due to imprecision
9. 95% CrIs of the network estimates include small benefit (42 fewer) to appreciable harm (137 more). Hence, the certainty of evidence was rated down by one level due to imprecision
10. In the direct comparison, the credible intervals included trivial benefit (7 fewer per 1000) to appreciable harm (191 fewer per 1000). Therefore, the certainty of evidence was rated down by one level for imprecision. 95% CrIs of the network estimates include small benefit (27 fewer) to very large harm (897 more). Hence, the certainty was rated down by two more levels due to very serious imprecision (implausible effect sizes) in the network estimates.
11. In the direct comparison, the certainty of evidence was rated down one level due to serious inconsistency; the certainty was further rated down one level for imprecision as the credible intervals include small benefit (33 fewer per 1000) to appreciable harm (111 more per 1000). Therefore, the overall certainty of evidence for the direct estimate was rated as low. No further change was made based on the network estimates.
12. In the direct comparison, the credible intervals include moderate benefit (86 fewer per 1000) to large harm (132 more per 1000). Therefore, the certainty of evidence was rated down by two levels for imprecision (as the confidence limits include appreciable benefit or harm). No further change was made based on the network estimates
13. In the direct comparison, the credible intervals include moderate benefit (60 fewer per 1000) to large harm (111 more per 1000). Therefore, the certainty of evidence was rated down by two levels for imprecision (as the credible intervals include appreciable benefit and harm). No further change was made based on the network estimates
14. In the direct comparison, the credible intervals include moderate benefit (59 fewer per 1000) to very large harm (797 more per 1000). Therefore, the certainty of evidence was rated down by two levels for imprecision (as the credible intervals include appreciable benefit and harm). The 95% CrIs of the network estimates include large benefit (109 fewer) to very large harm (583 more). Hence the certainty of evidence was rated down by one more level due to very serious imprecision (implausible effect sizes) in the network estimates
* A network absolute risk difference was calculated from the network RR estimates using an assumed control risk that was derived by dividing the total event number by the total infant number in the control groups in the network
**Comments on interpretation of effect sizes are based on a priori defined thresholds as follows: (a) For the outcome of mortality: Small benefit/harm was defined as <20 fewer or more per 1000, respectively. Moderate benefit/harm was defined as 20 to 50 fewer or more per 1000, respectively. Large benefit/harm was defined as >50 fewer or more per 1000 respectively; (b) For all other outcomes listed in the summary of findings table: Any effect <20 fewer or more per 1000 was defined as a trivial benefit or harm. No direction of effect was specified for trivial effects. Small benefit/harm was defined as 20‐50 fewer or more per 1000 respectively. Moderate benefit/harm was defined as 50‐100 fewer or more per 1000 respectively. Large benefit/harm was defined as >100 fewer or more per 1000, respectively. Language for interpretation used in this column is based on the GRADE informative statements to communicate the findings of systematic reviews of interventions by Santesso 2020.
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Background
Description of the condition
The most important contributors to morbidity and mortality in preterm infants are intraventricular haemorrhage (IVH), prolonged duration of endotracheal mechanical ventilation with consequent lung injury, and haemodynamic disturbance leading to compromised end‐organ perfusion (Clyman 2012; The Canadian Neonatal Network 2019). A common factor potentially responsible for these three pathophysiological mechanisms is patent ductus arteriosus (PDA) (Gournay 2011). The ductus arteriosus is a blood vessel that connects the aorta with the pulmonary artery to bypass the lungs during fetal life. Following birth, closure of the ductus arteriosus begins and functional closure occurs over the next 24 to 72 hours (Benitz 2016). In preterm infants, this process is usually delayed, leading to the ductus arteriosus remaining open beyond the first few days after birth. As a consequence, blood flow through the lungs increases and predisposes the infant to pulmonary congestion, surfactant inactivation, and respiratory failure, leading to increased oxygen requirement and need for ventilator support. At the same time, diversion of blood flow from the systemic circulation leads to systemic hypoperfusion of the bowel, kidney, and brain. Persistence of a PDA along with clinical signs of pulmonary congestion or systemic hypoperfusion (or both) is defined as a symptomatic or haemodynamically significant PDA. A persistent, symptomatic PDA in extremely preterm infants (infants born less than 28 weeks of gestational age) is associated with IVH and cerebral palsy, chronic lung disease, necrotizing enterocolitis (NEC), renal failure, and consequently higher rates of death (Ballabh 2010; Brown 1979; Chung 2005; Dice 2007; Dollberg 2005; Drougia 2007). According to The Canadian Neonatal Network 2019 report, 28% of preterm infants born at less than 33 weeks of gestation in Canada developed a PDA, and 48% of infants with a PDA received treatment with pharmacotherapy or surgical ligation.
Description of the intervention
Currently available pharmacotherapeutic options to prevent or treat a symptomatic PDA include cyclo‐oxygenase inhibitor (COX‐I) drugs such as indomethacin, ibuprofen, and acetaminophen (Mitra 2018). Indomethacin and ibuprofen are non‐steroidal anti‐inflammatory drugs (NSAIDs) that act by inhibition of the cyclo‐oxygenase enzyme, thereby leading to downregulation of prostaglandin E2, a potent relaxant of the PDA (Clyman 2012; Jain 2015). Recently, acetaminophen, a selective inhibitor of the cyclo‐oxygenase‐2 enzyme, has emerged as another treatment option for PDA closure (Le 2015). Acetaminophen is postulated to inhibit the peroxidase enzyme, resulting in downregulation of prostaglandin E2 production (Grèen 1989).
Use of indomethacin in preterm infants is associated with derangement of renal function (Seyberth 1983), NEC (Coombs 1991), gastrointestinal haemorrhage or perforation (Wolf 1989), alteration of platelet function (Friedman 1976), and impairment of cerebral oxygenation and blood flow (Ohlsson 1993). Ibuprofen appears to be associated with a lower risk of NEC and only transient renal insufficiency compared to indomethacin (Ohlsson 2020a). Acetaminophen has no documented short‐term adverse effects. However, recent observational studies have indicated a possible association of maternal acetaminophen exposure with later development of autism and attention deficit/hyperactivity disorder (Bauer 2013; Ji 2020; Ystrom 2017).
This review focuses on the prophylactic use of COX‐I drugs (indomethacin, ibuprofen, or acetaminophen) to prevent death and PDA‐related morbidities in preterm infants.
How the intervention might work
The aim of prophylactic COX‐I drugs is to close a PDA before the development of any adverse haemodynamic consequences but without the need for echocardiographic screening or surveillance. In addition to PDA closure, prophylactic COX‐I drugs may also directly affect the cerebral vasculature to prevent occurrence of IVH.
All available COX‐I drugs (indomethacin, ibuprofen, and acetaminophen) have been shown to be significantly more effective in closing a PDA compared to no treatment (Mitra 2018). Ibuprofen appears as effective as indomethacin in closing a PDA (Ohlsson 2020a). There is moderate‐certainty evidence to suggest that acetaminophen is as effective as ibuprofen and low‐certainty evidence to suggest that acetaminophen is as effective as indomethacin in closing a PDA (Ohlsson 2020b).
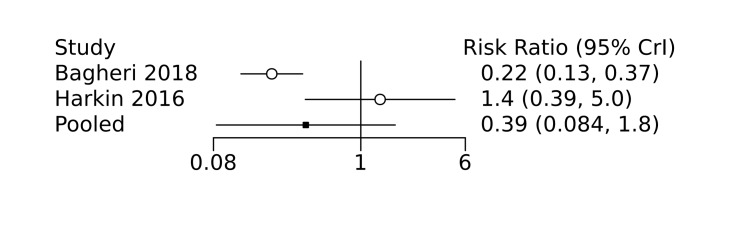
With regards to effect on the cerebral vasculature, Ment 1992 demonstrated in animal models that indomethacin stimulates basement membrane deposition in the germinal matrix microvessels that may prevent germinal matrix haemorrhage and IVH. This postulated reduction in IVH has subsequently been demonstrated through randomized controlled trials (RCTs) of prophylactic indomethacin in preterm infants (Fowlie 2010). Prophylactic ibuprofen has also been shown to marginally reduce the incidence of severe IVH (Ohlsson 2020c). The role of acetaminophen in reduction of IVH in preterm infants has not yet been clearly established. IAcetaminophen may help to prevent IVH by decreasing harmful mitochondrial superoxide production and intracellular oxidant stress, in addition to its direct effect on ductal constriction (Härmä 2020). In the post‐hoc analysis of a recent RCT of prophylactic acetaminophen in very preterm infants (Härkin 2016), it was shown that infants in the acetaminophen group had a significantly higher ductal closure, significantly higher peripheral oxygen saturation (SpO2), significantly higher regional cerebral oxygen saturation (RcSO2), and significantly lower cerebral fractional tissue oxygen extraction (cFTOE) during the treatment period compared to the control group (Härmä 2020). This effect might be a direct effect of ductal constriction and improved cerebral blood flow, or an effect at the cellular level whereby acetaminophen reduced cFTOE by reducing mitochondrial respiration (Bisaglia 2002; Vergeade 2016). Several previous studies have shown that occurrence of IVH in preterm infants is preceded by reduction in RcSO2 and increase in cFTOE (Baik 2015; Cimatti 2020). Therefore, by improving RcSO2 and reducing cFTOE, acetaminophen may help to prevent IVH in preterm infants.
Although PDA and IVH are common morbidities in preterm infants, the clinical use of pharmacoprophylaxis has been a contentious issue. As discussed above, evidence from RCTs suggests that prophylactic use of indomethacin or ibuprofen could reduce severe IVH in preterm infants (Fowlie 2010; Ohlsson 2020c), but may unnecessarily expose a large number of preterm infants to the harmful effects of COX‐I drugs (Fowlie 2010; Reese 2017; Stavel 2017).
Why it is important to do this review
The clinical use of pharmacoprophylaxis has primarily been driven by the perceived benefits versus potential risks, as determined by the treating physician. Successful prevention of a symptomatic PDA may reduce the risk of severe IVH, chronic lung disease, and death, but at the same time may increase the risk of adverse outcomes. As a result, for some care providers the desirable consequences of COX‐I prophylaxis may not sufficiently outweigh its undesirable consequences, and hence there is often a reluctance among neonatal practitioners to consider pharmacoprophylaxis for PDA in preterm infants (Reese 2017; Stavel 2017). The thresholds for using COX‐I prophylaxis may also vary based on the balance of desirable and undesirable effects of each COX‐I drug.
Previous Cochrane Reviews have separately compared placebo/no treatment against prophylactic indomethacin, ibuprofen, or acetaminophen (Fowlie 2010; Ohlsson 2020b; Ohlsson 2020c). There are currently no Cochrane Reviews that provide head‐to‐head comparisons between the three available pharmacoprophylactic agents. With increased emphasis on non‐pharmacological conservative management, no prophylactic treatment has also become an increasingly adopted management approach. Given that there are currently four different management options (indomethacin, ibuprofen, acetaminophen, and no prophylaxis) available systematic reviews and meta‐analyses using paired comparisons provide care providers with limited evidence for informed decision‐making, which likely leads to substantial practice variation. For example, the Cochrane Review by Fowlie and colleagues demonstrated that prophylactic indomethacin reduces severe IVH with a risk ratio (RR) of 0.66 (95% confidence interval [CI] 0.53 to 0.82) compared to placebo (Fowlie 2010). Similarly, the review by Ohlsson and colleagues demonstrated that ibuprofen may marginally reduce severe IVH (RR 0.67 [95% CI 0.45 to 1.00]) (Ohlsson 2020c). However, it is difficult to conclude which drug is better in preventing severe IVH from these two separate analyses. Using network meta‐analysis to directly and indirectly compare available pharmacoprophylactic options may provide care providers with more reliable comparative effectiveness evidence with increased precision to help them choose the best available management option. Therefore, a systematic review and network meta‐analysis according to Cochrane methodology is justified.
Objectives
To determine the comparative effectiveness and safety of prophylactic cyclo‐oxygenase inhibitor (COX‐I) drugs (indomethacin, ibuprofen, or acetaminophen) and 'no COX‐I prophylaxis' in preterm infants using a Bayesian network meta‐analysis.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomized controlled trials (RCTs), irrespective of language and year of publication. Both superiority trials and non‐inferiority trials were eligible for inclusion. Unpublished RCTs were only included if the study authors agreed to provide details of the trial methodology so that the internal validity of the study could be adequately ascertained.
Types of participants
We included neonates that are preterm (born at less than 37 weeks’ completed gestation) or of low birth weight (less than 2500 grams). Given that we intended to perform a network meta‐analysis in this review, the transitivity assumption was strictly considered in the eligibility criteria. Only preterm or low birth weight infants, within the first 72 hours of birth and without a prior clinical or echocardiographic diagnosis of patent ductus arteriosus (PDA), were eligible for inclusion in the network meta‐analysis (for details, see Assessment of heterogeneity).
Types of interventions
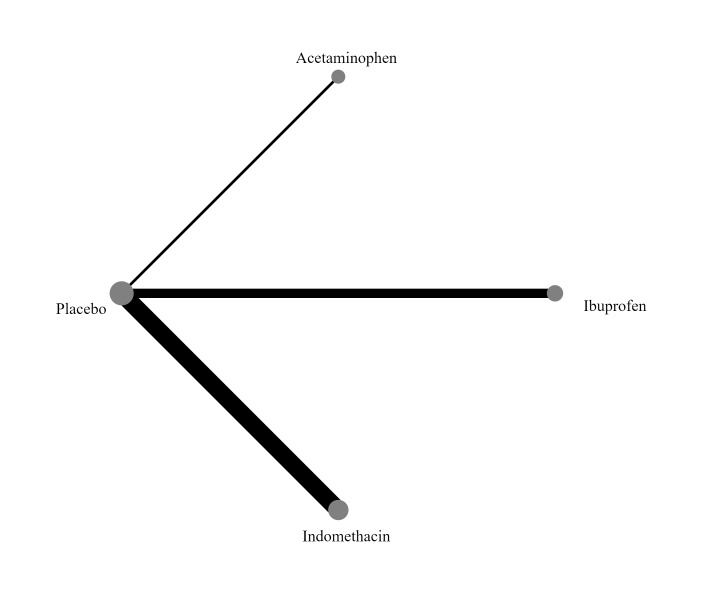
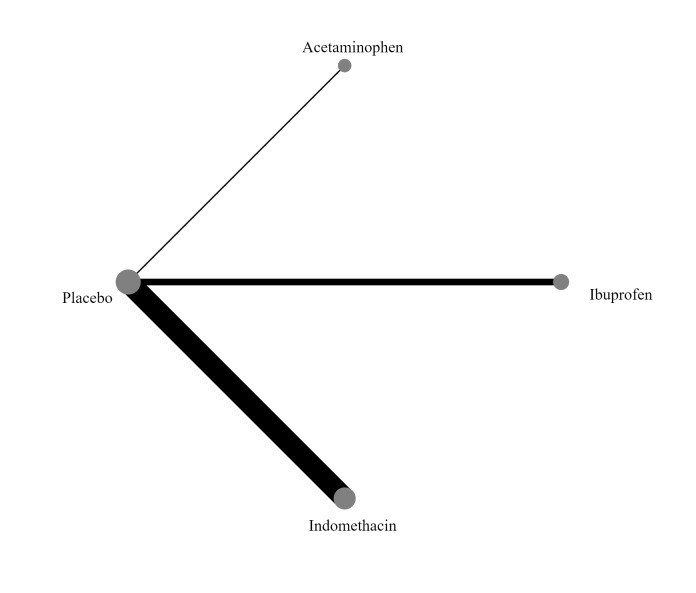
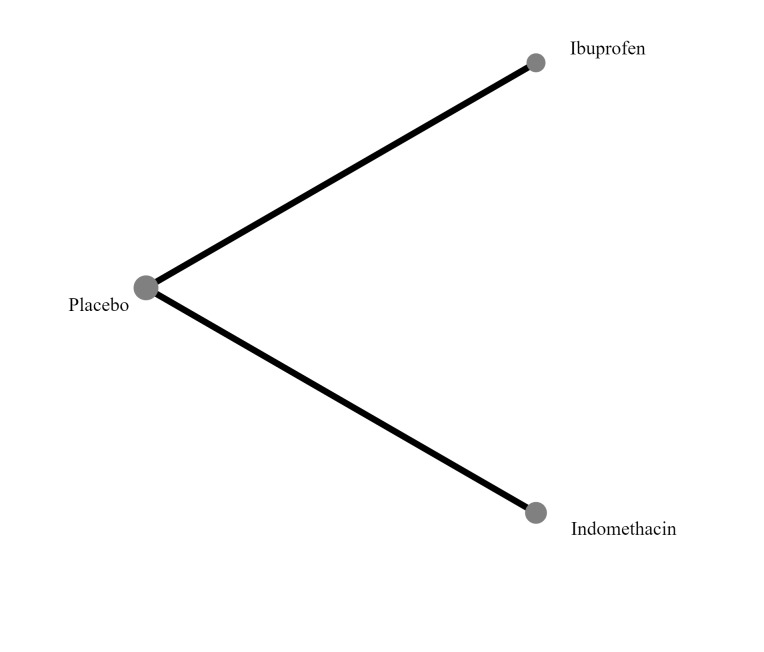
Interventions included prophylactic administration of indomethacin, ibuprofen, or acetaminophen, compared with active medication, placebo, or no prophylaxis. The intervention must be delivered within the first 72 hours after birth, and there must be no documented clinical or echocardiographic evidence of PDA. In the network meta‐analysis, each node was defined by the type of COX‐I (indomethacin, ibuprofen, or acetaminophen), or no prophylaxis.
A standard course of prophylactic indomethacin constituted a cumulative dosage of up to 0.6 mg/kg (Fowlie 2010). A standard course of prophylactic ibuprofen constituted a cumulative dosage of up to 20 mg/kg (Ohlsson 2020c). A standard course of prophylactic acetaminophen constituted a cumulative dosage of up to 420 mg/kg (15 mg/kg at six‐hour intervals for three to seven days) (Ohlsson 2020b). The nodes representing each medication in the network corresponded to these standard doses unless otherwise specified. If one or more of the included studies reported that cumulative doses for any of these medications were higher than the standard cumulative doses as mentioned above, separate nodes denoting higher cumulative doses of the medications were planned to be added to the network.
Types of outcome measures
Primary outcomes
Severe intraventricular haemorrhage (IVH) (grade 3 or 4) (Papile 1978)
Mortality (at discharge or at last reported follow‐up, whichever is later)
Secondary outcomes
Receipt of pharmacotherapy for symptomatic PDA
Surgical or interventional PDA closure
Necrotizing enterocolitis (NEC) (stage 2 or greater) (Bell 1978)
Gastrointestinal perforation (defined clinically by the presence of pneumoperitoneum in the absence of pneumatosis intestinalis and portal venous air on abdominal radiograph, and postoperatively by presence of isolated bowel perforation in the setting of an otherwise normal bowel, which is confirmed by histopathologic examination) (Meyer 1991; Pumberger 2002)
Chronic lung disease (CLD) (defined as use of oxygen or respiratory support at 36 weeks’ postmenstrual age) (Ehrenkranz 2005)
Oliguria (defined as urine output of less than 1 mL/kg/hour)
IVH of any grade (Papile 1978)
Periventricular leukomalacia (PVL; any grade) (de Vries 1992)
Neurodevelopmental outcome (at 18 to 24 months of age)
Cerebral palsy
Major neurodevelopmental disability, defined as the presence of any of the following: cerebral palsy, developmental delay (an assessment greater than two standard deviations [SDs] below the mean on the following scales: Bayley Scales of Infant Development ‐ Mental Development Index Edition II [BSID‐MDI‐II; Bayley 1993], Bayley Scales of Infant and Toddler Development ‐ Edition III Cognitive Scale [BSITD‐III; Bayley 2005] or Griffiths Mental Development Scale ‐ General Cognitive Index [GCI; Griffiths 1954; Griffiths 1970]), intellectual impairment (intelligence quotient [IQ] greater than two SDs below the mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013).
Search methods for identification of studies
An Information Specialist (RP) developed search strategies in consultation with the authors. Leah Boulos peer‐reviewed the MEDLINE search. Methodological filters were used to limit retrieval to randomised controlled trials. Searches for trials were conducted without language, publication year, publication type, or publication status restrictions. Methodological filters were sourced from the Cochrane Handbook of Systematic Reviews and the ISSG Search Filters Resource (https://sites.google.com/a/york.ac.uk/issg-search-filters-resource/home).
Trial registries and conference abstracts were searched. Authors checked the reference lists of related systematic reviews and studies.
Electronic searches
The following databases were searched in December 2021.
Cochrane Central Register of Controlled Trials (CENTRAL), 9 December 2021(via Wiley, 2021, Issue 12,)
Ovid MEDLINE(R) ALL <1946 to 8 December 2021>
Embase 1974 to 9 December2021 (Elsevier)
Epistemonikos (https://www.epistemonikos.org)
MEDLINE, Embase and CENTRAL search strategies are available in Appendix 1
Searching other resources
Trial registration records were identified using Cochrane CENTRAL and by independent searching of the following:
· U.S. National Library of Medicine registry (clinicaltrials.gov);
· World Health Organization’s International Trial Registry and Platform (https://www.who.int/clinical-trials-registry-platform);
· The ISRCTN Registry (https://www.isrctn.com/).
Trial registry search strategies are available in Appendix 1.
Conference abstracts were identified using CENTRAL, Embase and via the following websites: • The European Society for Pediatric Research: https://www.espr.eu/ • Pediatric Academic Societies: https://www.pas-meeting.org/past-abstracts/
We checked the reference lists of included studies and the reference lists of related systematic reviews to identify studies not captured in database searches. We searched for errata or retractions for included studies published on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Pairs of review authors (SM, AM, DS, CEG) independently screened the search results by title and abstract for studies that potentially met the inclusion criteria. We obtained the full text of any articles that were potentially eligible, and two review authors independently performed full‐text assessments (SM, AM, CEG). We resolved any disagreements through discussion and consensus. In the absence of consensus, a third person adjudicated on the decision for inclusion or exclusion of studies. We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009) and to complete 'Characteristics of included studies' and 'Characteristics of excluded studies' tables. We carried out the study selection process on the Covidence platform.
Data extraction and management
Three review authors (SM, AM, CEG) independently extracted, assessed, and coded all data for each study using a standardized, piloted form developed in Microsoft Excel. We resolved any disagreements through consensus. For each study, one review author (SM) entered the extracted data into the GEMTC GUI application (van Valkenhoef 2012), and a second review author (CEG) checked data entry. We collected information regarding the following.
General information: name of review author carrying out data extraction; study ID (and any other unique trial identifiers); name and contact address of first/corresponding author of included trial; citation of included trial; language of trial and details of any duplicate publications.
Trial information: trial design (type of RCT); location of trial; setting; sample size; study duration; treatment arms; method of randomization; inclusion and exclusion criteria; length of follow‐up; trial registration data.
Characteristics of participants: gestational age; birth weight; baseline characteristics (sex; mode of delivery; receipt of antenatal steroids; deferred cord clamping); age (in hours) at initiation of treatment.
Characteristics of interventions: number of treatment arms; description of experimental and control arm(s); timing, dose and route of administration of intervention; other differences between intervention arms.
Outcomes: all relevant arm‐level data on primary and secondary outcomes as outlined in Types of outcome measures. We will also collect data on stated outcome measures that have been defined in a manner different from our stated definitions in Types of outcome measures.
Risk of bias: sequence generation; allocation concealment; blinding (participants, personnel, outcome assessors); incomplete outcome data; selective outcome reporting; other sources of bias.
We also intended to collect data on any cost or resource information reported in the included studies. Although this does not constitute a formal economic evaluation, it may provide useful additional information that may be of value in development of a clinical practice guideline. If information was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (SM, AM, CEG) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias tool for the following domains (Higgins 2019).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
We resolved any disagreements by consensus. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
Relative treatment effects
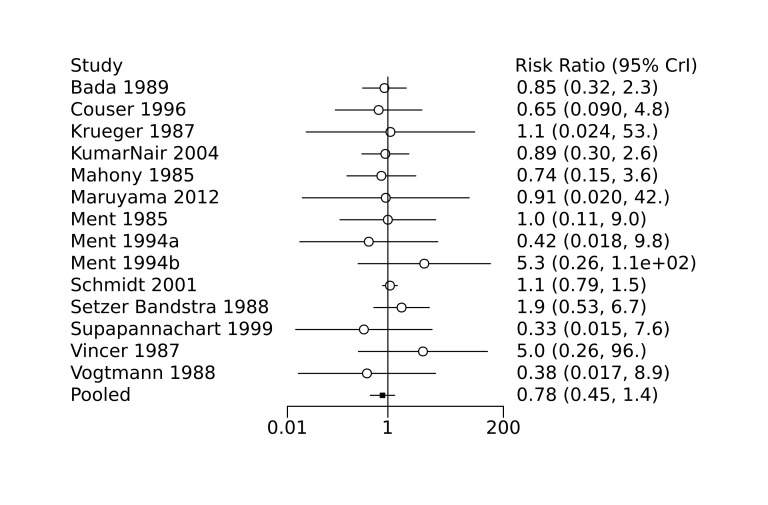
We used risk ratios (RRs) and absolute risk differences (ARDs) for categorical variables, and mean differences (MDs) for continuous variables. We used Bayesian random‐effects models with a binomial likelihood and log link for both initial pairwise meta‐analyses as well as subsequent network meta‐analyses (see Data synthesis for details). Therefore, we reported the 95% credible intervals (CrIS) for all estimates. These were summarized in forest plots displaying the results from pairwise, indirect and network (combining direct and indirect) analyses for the comparisons of treatment with one COX‐I medication (indomethacin, ibuprofen, acetaminophen) versus another or control (placebo or no treatment). A network ARD was calculated from the network RR estimates using an assumed control risk that was derived by dividing the total event number by the total infant number in the control groups in the network.
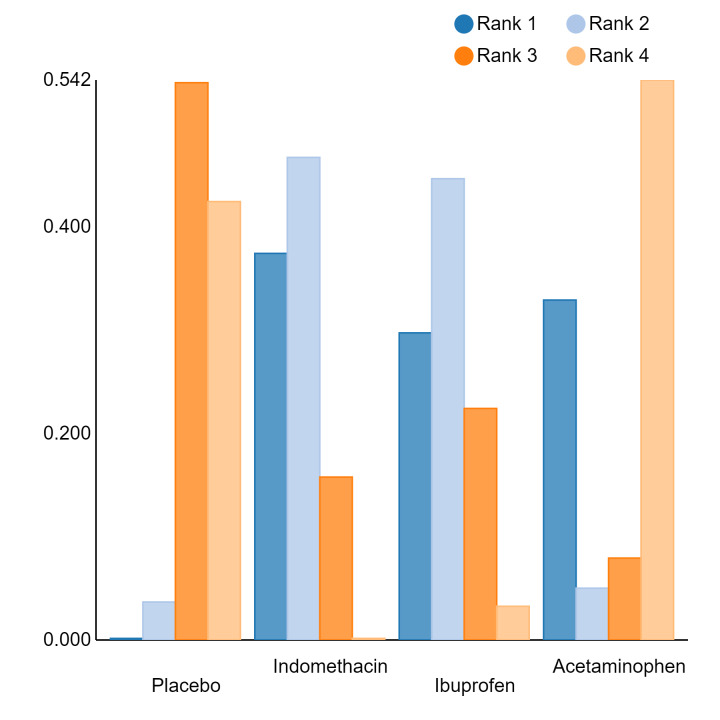
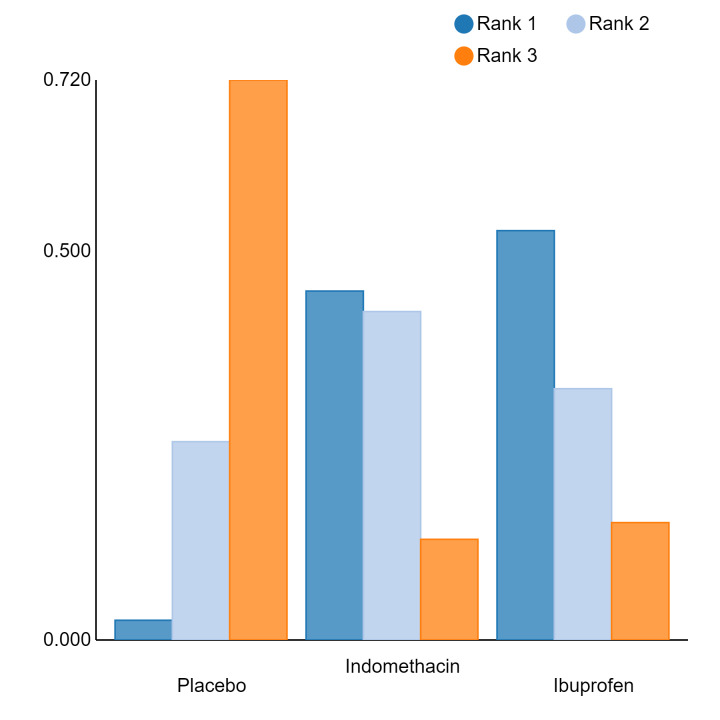
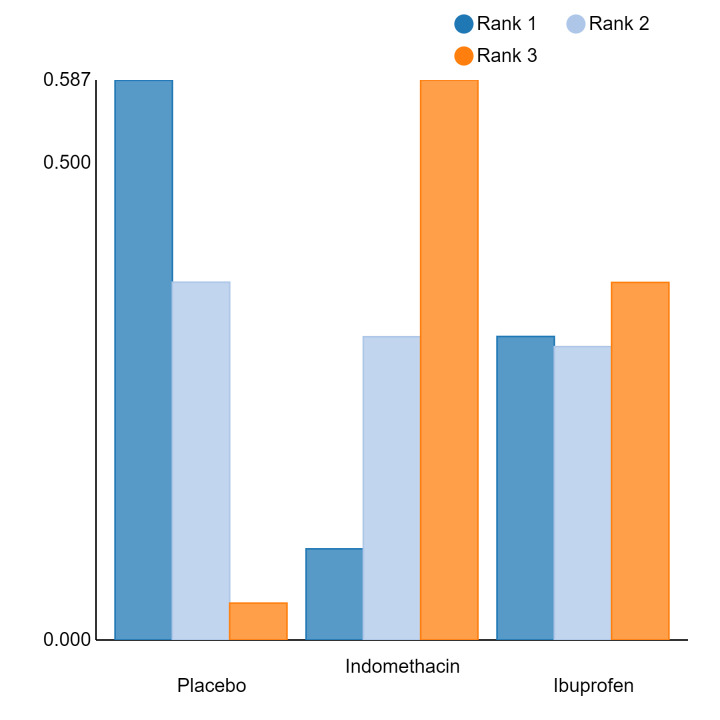
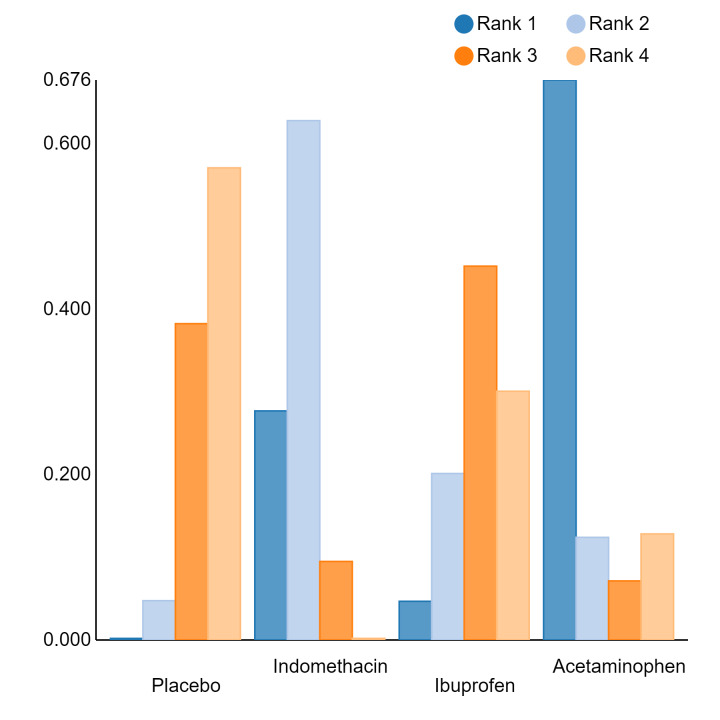
Relative treatment ranking
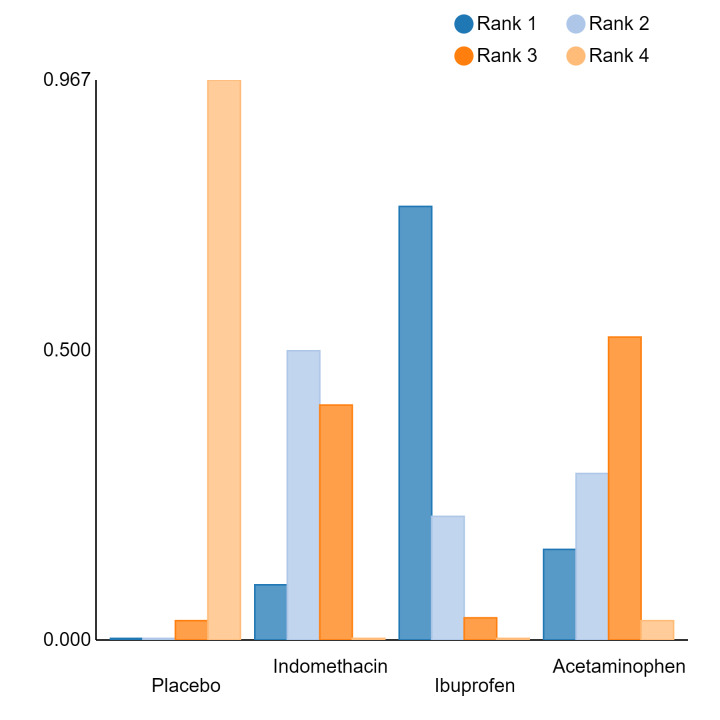
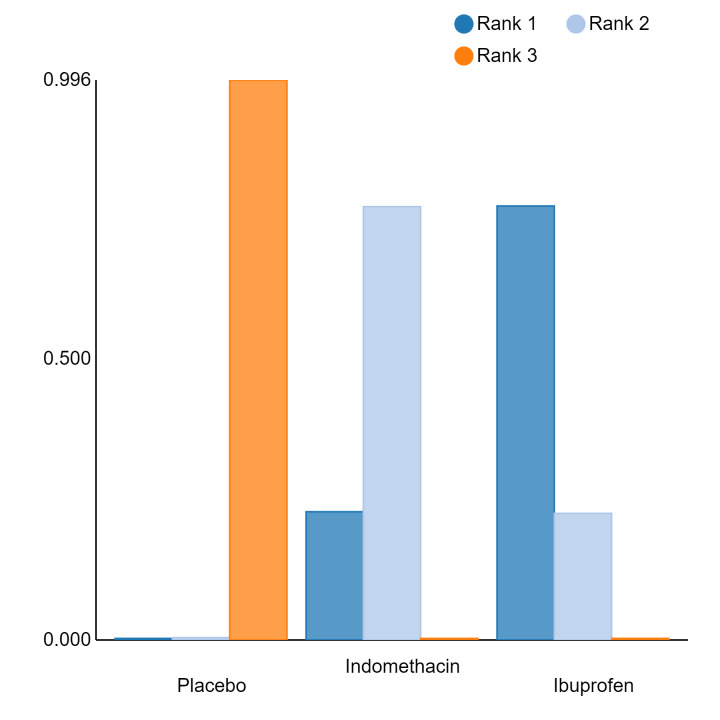
An overall ranking for each intervention was built from these RRs and was presented as median ranks (with 95% CrIs) for each outcome. We further calculated the surface under the cumulative ranking curve (SUCRA) to explore the potential order of treatment hierarchy (Salanti 2011). SUCRA is an index reflecting the degree to which an intervention is superior or inferior to the others. Calculation of SUCRA is based on the cumulative probabilities of the treatments being ranked in each position, and the SUCRA is the final area under the curve of the graph for these probabilities. SUCRA would be one when a treatment is certain to be the best and zero when a treatment is certain to be the worst with values ranging from one (the best intervention) to zero (the worst intervention).
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials. We included multi‐arm trials, and accounted for the correlation between the effect estimates in the network meta‐analysis (NMA). We treated multi‐arm studies as multiple independent comparisons in pairwise meta‐analyses and these were not combined in any analysis.
For cluster‐RCTs, if studies had not taken clustering into account, methods in the Cochrane Handbook of Systematic Reviews of Interventions were used to perform approximately correct analyses (Higgins 2019). Data from cluster‐randomized trials were only included in meta‐analyses if clustering had been quantified and reported using an intra‐cluster correlation coefficient (ICC), or if other approximately correct analyses could be performed (Costantini 2020). For cross‐over RCTs, data from only the first period prior to cross‐over were used, due to potential carry‐over effects.
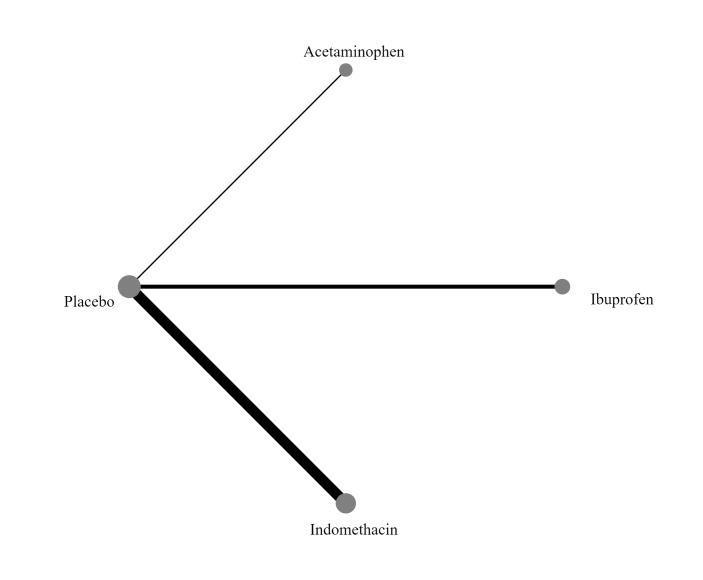
'No prophylaxis' was included as a node in the NMA to help with indirect analyses and formation of a hierarchy of interventions. In the NMA, we included all comparisons where there are sufficient data to do so.
Dealing with missing data
We handled missing data according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). For included studies, we recorded the number of participants lost to follow‐up. We contacted corresponding authors to obtain any missing participant outcome data that were not reported. We attempted to contact the authors up to a maximum of three times to obtain missing information. If we were still unable to obtain the missing outcome information, and where missing data were thought to introduce serious bias (defined as 20% or greater missing data), we performed sensitivity analysis to evaluate the impact of missing outcome data. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. all participants will be analyzed in the group to which they are allocated, regardless of whether or not they receive the allocated intervention).
Assessment of heterogeneity
Assessment of clinical and methodological heterogeneity within treatment comparisons
Prior to synthesis, we assessed all studies for clinical and methodological differences that may give rise to heterogeneity. We only pooled data if the studies were judged to be sufficiently similar from a clinical and methodological perspective.
Assessment of transitivity across treatment comparisons
We defined transitivity as the assumption that the studies were sufficiently similar in their distribution of effect modifiers on average so that indirect comparisons could be used as a valid method to compare two treatment options (Baker 2002; Cipriani 2013; Donegan 2010).
Transitivity was established if the included infants met the following criteria with respect to potential effect modifiers.
Gestational age and birth weight: all infants included in the NMA had a gestational age at birth of less than 37 weeks, or a birth weight of less than 2500 g (or both)
PDA status: all included infants were randomized to receive the intervention(s) prophylactically, and not based on prior clinical/echocardiographic knowledge of their PDA
Timing of intervention: all included infants received the interventions within the first 72 hours after birth
Investigation of heterogeneity
We explored statistical heterogeneity in both pairwise and network comparisons. In case of pairwise comparisons, we assessed the heterogeneity by visual inspection of the forest plots and by using the I2 statistic, with the following thresholds for interpretation (Higgins 2019).
Less than 25%: no heterogeneity
25% to 49%: low heterogeneity
50% to 74%: moderate heterogeneity
Greater than 75%: substantial heterogeneity
Assessment of statistical inconsistency
Evidence from an NMA may be inconsistent if the direct and indirect evidence is incompatible (loop inconsistency) or the studies involving one of the treatments are fundamentally different from the studies involving another treatment (design inconsistency) (White 2012). The consistency assumption among the combined sources of evidence in the network was first evaluated globally for the entire network using the design × treatment interaction model (Dias 2010; White 2012). We then applied the node‐splitting model to assess local inconsistency for each comparison. In the node‐splitting analysis a treatment comparison was split into a parameter for direct evidence and a parameter for indirect evidence in order to assess whether there was a significant disagreement between the two parameters. A P value of less than 0.05 indicated significant incoherence between the direct and indirect comparisons (Dias 2010; van Valkenhoef 2012; Veroniki 2013; White 2012). A common within‐network heterogeneity was assumed as the treatments were of similar nature, belonging to the same class of drugs (COX‐I drugs) (Mitra 2018).
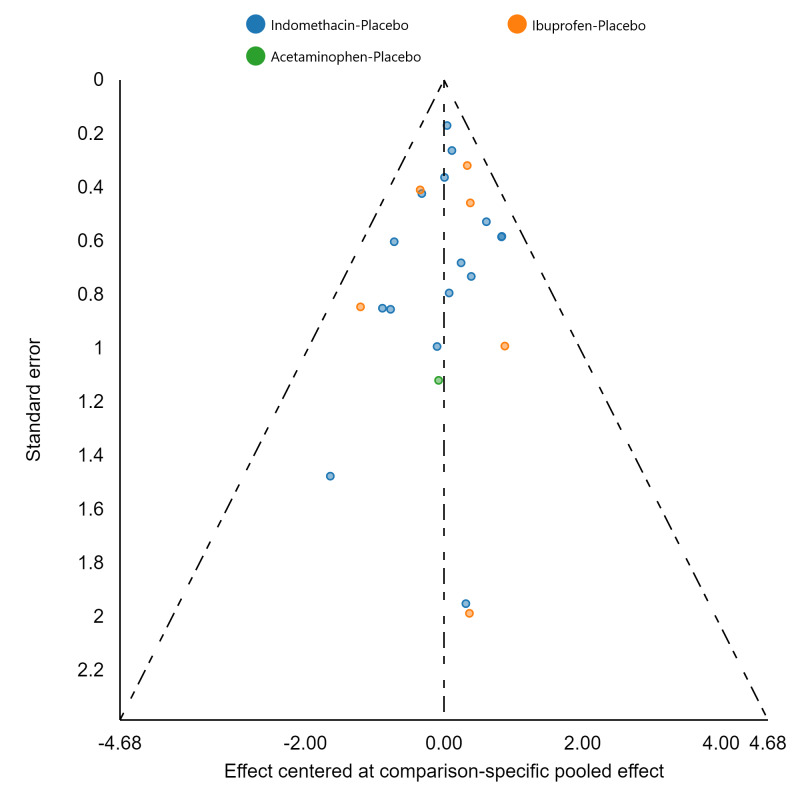
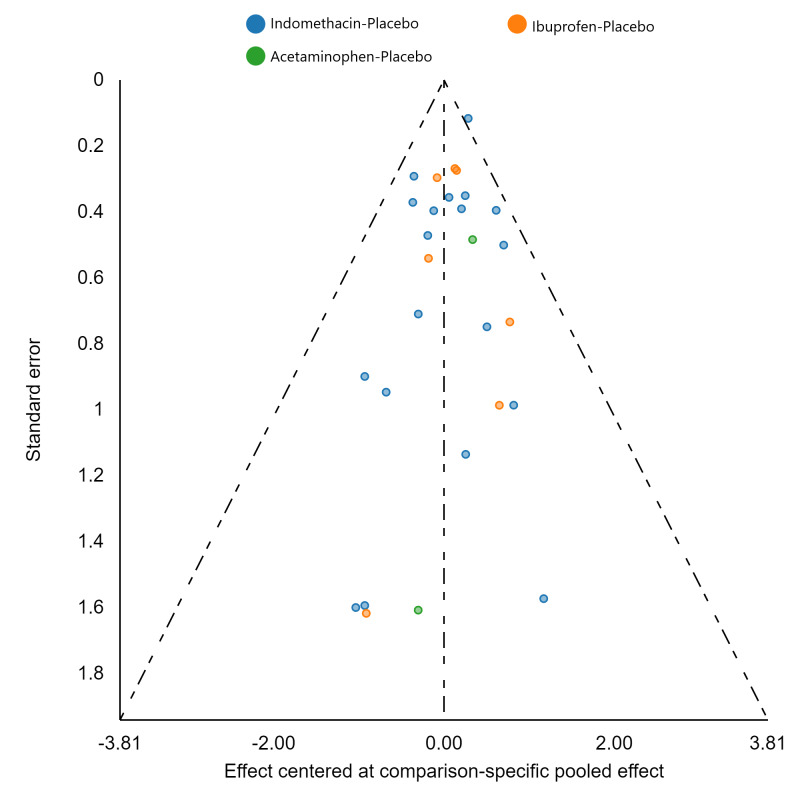
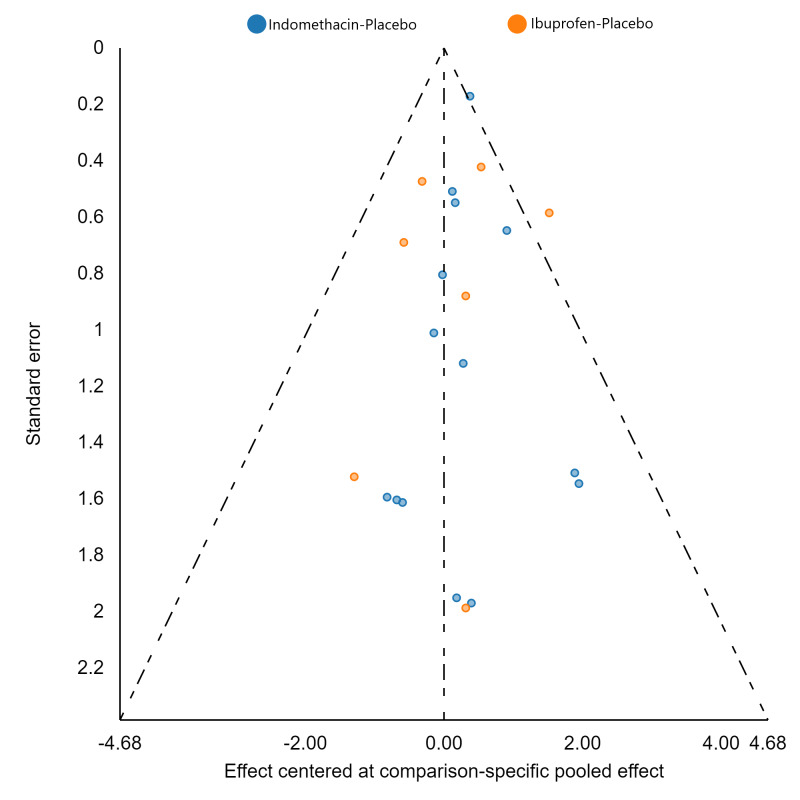
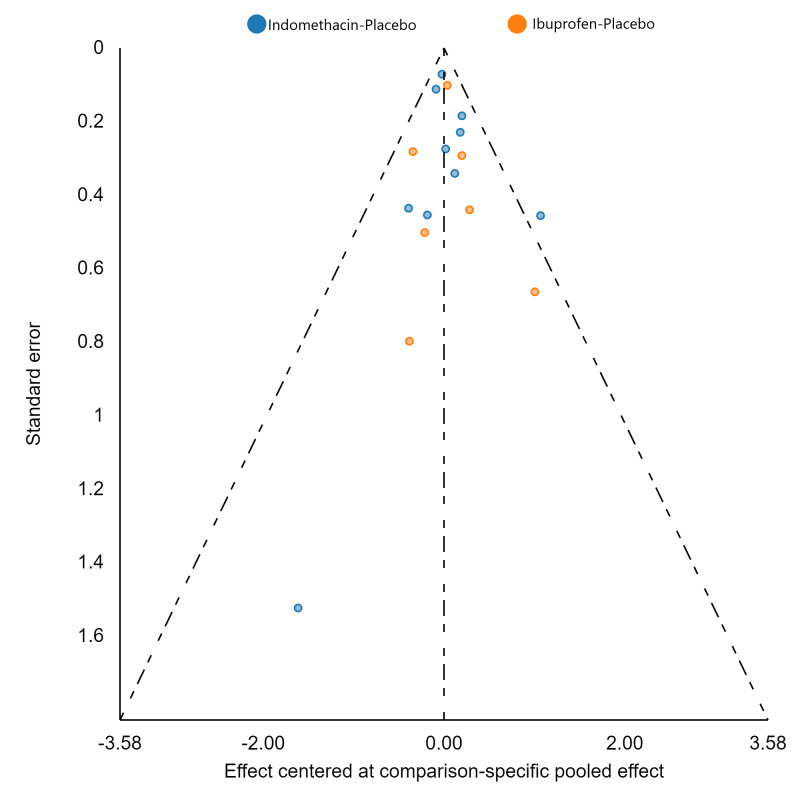
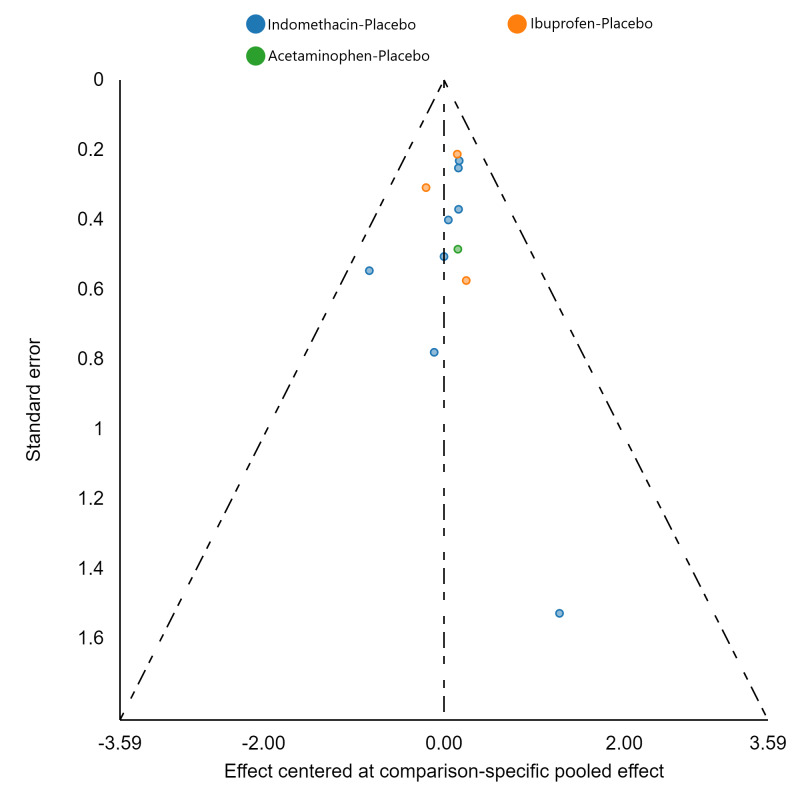
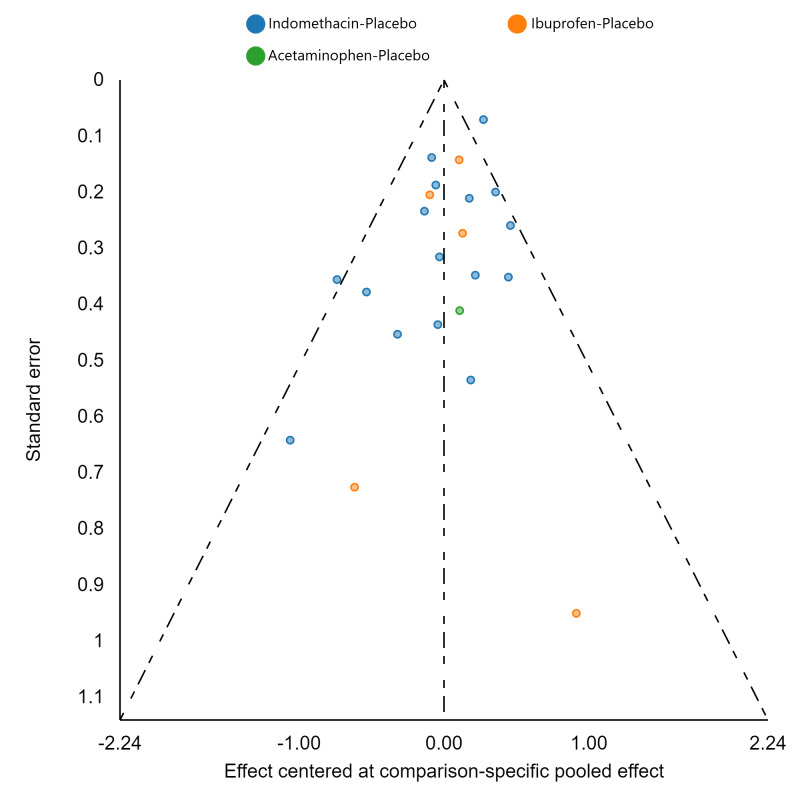
Assessment of reporting biases
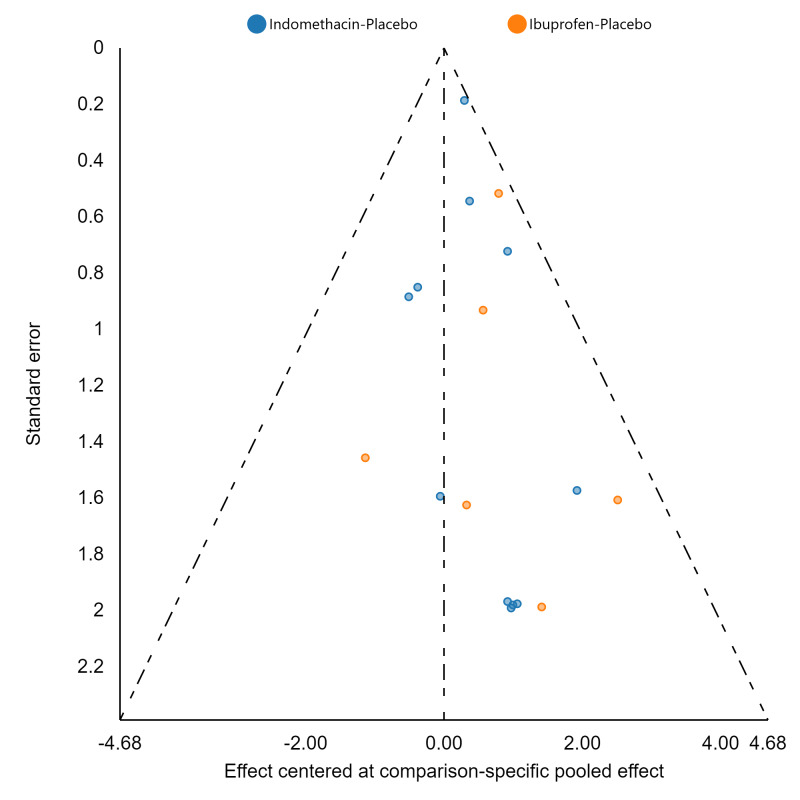
If there were 10 or more studies in a pairwise meta‐analysis, we explored the existence of small‐study effects (publication bias) through visual inspection of comparison‐adjusted funnel plots (Dias 2013; van Valkenhoef 2012). In addition, we evaluated whether results of published posters and available dissertations were subsequently published as full‐length manuscripts. We identified records in trial registries that have been terminated, listed as complete, or should feasibly be complete given last updated status with regard to availability of results or subsequent publication. For preregistered trials or those with published protocols, we assessed for the presence of reporting bias through comparison of their preplanned primary and secondary outcomes and analysis methods against those reported and used in the published report.
Data synthesis
We performed the network meta‐analysis (NMA) following the methods stated in the Cochrane Handbook for Systematic Reviews of Interventions for all outcome measures (if data were available) (Higgins 2019).
For each outcome, we performed initial pairwise meta‐analysis using a Bayesian random‐effects model for every direct pairwise comparison, where applicable. We then performed a Bayesian random‐effects NMA to compare all interventions simultaneously using the Markov chain Monte Carlo method conducted under the assumption of transitivity (see Assessment of heterogeneity) (Lambert 2005; Lu 2004). We further assessed the inconsistency between the direct and indirect estimates, first globally for the entire network using the design × treatment interaction model, and then locally for each comparison using the node‐splitting model (see Assessment of heterogeneity) (Dias 2010; van Valkenhoef 2012; Veroniki 2013; White 2012).
For both pairwise meta‐analysis and the NMA, we used Bayesian hierarchical models with non‐informative priors assigned to all model parameters. Prior distributions for the relative effects were determined heuristically based on the following: N(0, (15 ⋅ S )2), where N denotes normal distribution and S denotes the outcome scale. The value of S corresponded to an implausibly large variation on the scale of analysis which was determined heuristically based on available data (van Valkenhoef 2012). We used a series of 100,000 simulations to allow convergence and, after thinning of 10 and discarding the first 20,000 simulations, produced the outputs. We assessed model convergence on the basis of Gelman and Rubin diagnostic tests (Gelman 1992; Mitra 2018). We planned to conduct all analyses (both pairwise meta‐analyses and NMA) using the R (R Core Team 2020) package gemtc on the MetaInsight application (Owen 2019), developed by the Cochrane Complex Review Support Unit (CRSU). We planned to conduct the design × treatment model to assess global network inconsistency will be performed in Stata version 15 (StataCorp) using the network command or similar software (Palmer 2016).
Subgroup analysis and investigation of heterogeneity
If the information was available we planned to conduct subgroup analyses for the following factors, to explore potential effect modification.
Gestational age (less than 28 weeks versus 28 weeks or greater)
Birth weight (less than 1000 g versus 1000 g or more)
Initiation of prophylaxis (24 hours of age or less versus over 24 hours of age)
Based on available information, we planned subgroup analyses for the following outcomes.
Severe IVH (grade 3 or 4) (Papile 1978)
Mortality (at discharge or last reported follow‐up, whichever is later)
Surgical or interventional PDA closure
NEC (stage 2 or greater) (Bell 1978)
Gastrointestinal perforation (Meyer 1991; Pumberger 2002)
Chronic lung disease (CLD) (defined as use of oxygen or respiratory support at 36 weeks’ postmenstrual age) (Ehrenkranz 2005)
Major neurodevelopmental disability
We planned to assess subgroup differences by comparing the network diagram for each subgroup. We then planned to perform a pairwise and NMA for each subgroup, and compare their relative treatment effects and their relative treatment ranking.
Sensitivity analysis
We planned to conduct sensitivity analyses to determine whether the findings were affected by including only studies of adequate methodology (low risk of bias), defined as those studies with adequate randomization and allocation concealment, blinding of intervention and measurement, and up to and including a 20% loss to follow‐up.
Based on available information, sensitivity analyses were planned for the following outcomes.
Severe IVH (grade 3 or 4) (Papile 1978)
Mortality (at discharge or last reported follow‐up, whichever is later)
Surgical or interventional PDA closure
NEC (stage 2 or greater) (Bell 1978)
Gastrointestinal perforation (Meyer 1991; Pumberger 2002)
CLD (defined as use of oxygen or respiratory support at 36 weeks’ postmenstrual age) (Ehrenkranz 2005)
Major neurodevelopmental disability
Network meta‐regression
We anticipated that RCTs on prophylactic use of COX‐I drugs would have been conducted over the last 40 years, and would encompass wide variation in neonatal intensive care practices which was otherwise difficult to document as co‐interventions or possible effect modifiers. Therefore, for each network, if at least 10 studies were available, we conducted a network meta‐regression, assuming a common fixed coefficient across comparisons to explore the effect of year of publication on the most important clinical outcomes, i.e. mortality, severe IVH, gastrointestinal perforation, NEC, and CLD (Mitra 2018). We assumed year of publication as a proxy for contemporary neonatal care practices.
Summary of findings and assessment of the certainty of the evidence
We made an assessment of our confidence in the estimates (certainty of evidence) according to the GRADE criteria for NMA, as outlined by the GRADE working group (Brignardello‐Petersen 2018; Puhan 2014), for the following outcomes.
Severe IVH (grade 3 or 4) (Papile 1978)
Mortality (at discharge or last reported follow‐up, whichever is later)
Surgical or interventional PDA closure
NEC (stage 2 or greater) (Bell 1978)
Gastrointestinal perforation (Meyer 1991; Pumberger 2002)
CLD (defined as use of oxygen or respiratory support at 36 weeks’ postmenstrual age) (Ehrenkranz 2005)
Major neurodevelopmental disability, defined as the presence of any of the following: cerebral palsy, developmental delay (Bayley Scales of Infant Development ‐ Mental Development Index Edition II [BSID‐MDI‐II; Bayley 1993], Bayley Scales of Infant and Toddler Development ‐ Edition III Cognitive Scale [BSITD‐III; Bayley 2005] or Griffiths Mental Development Scale ‐ General Cognitive Index [GCI; Griffiths 1954; Griffiths 1970] assessment greater than two standard deviations [SDs] below the mean), intellectual impairment (intelligence quotient [IQ] greater than two SDs below the mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013).
To assess the certainty of evidence in a network meta‐analysis, we took both direct and indirect comparisons into account (Brignardello‐Petersen 2018; Puhan 2014). We assessed the certainty of evidence for each pairwise comparison using the following steps.
Certainty of evidence from the direct comparison, if available (step 1): We assessed and rated the direct comparison between two interventions (if head‐to‐head RCT data are available) based on the following categories, as outlined in the GRADE Handbook (Guyatt 2008; Schünemann 2013): risk of bias; indirectness; inconsistency (which is determined based on the heterogeneity assessment for pairwise comparisons); imprecision; and publication bias.
Certainty of evidence from the indirect comparisons (step 2): We followed step 1 for assessment of confidence from indirect estimates. For rating confidence in the indirect comparisons, we used the information obtained from the first‐ and second‐order loops in the network. We preferentially derived the certainty of evidence of indirect comparisons from the certainty of evidence of the first‐order loops. We derived the certainty of evidence of a first‐order loop from the lowest certainty of evidence among direct comparisons within the first‐order loop. When an indirect comparison has two or more first‐order loops, we used the highest certainty of evidence among its first‐order loops for the certainty of evidence of the indirect comparison. When no first‐order loop was available, we derived the certainty of evidence for an indirect comparison from the second‐order loops (Puhan 2014).
Overall certainty of evidence for the comparison from the NMA (step 3): We rated the overall certainty in the NMA estimates for any paired comparison using the higher of the certainty rating amongst the contributing direct and indirect comparisons, if no statistically significant incoherence was observed. The specific reason for taking the higher certainty of evidence between the two comparisons was that if the direct and indirect estimates were coherent, the estimate with the lower certainty was not likely to introduce bias relative to the estimate with the higher certainty. If statistically significant incoherence was observed between the direct and indirect estimates, then the certainty of evidence for the comparison that made a dominant contribution to the network estimate was taken as the overall certainty of evidence. We determined the dominant contribution from the 95% CrI of the forest plots for the direct and indirect comparisons. The comparison that had the narrower 95% CrI between the two would have had the dominant contribution to the network (Brignardello‐Petersen 2018).
Assessment of inconsistency (step 4): If inconsistency was noted either for the entire network using the design × treatment interaction model, or locally for each comparison using the node‐splitting model (or both), we rated the certainty in the NMA estimate down by one level. When assessment of statistical inconsistency was not possible due to absence of head‐to‐head comparisons between interventions, we did not rate down the certainty of evidence any further due to presumed inconsistency, as the NMA would have been conducted under the strict assumption of transitivity thereby ensuring clinical and methodological homogeneity between the indirect comparisons.
Assessment of imprecision (step 5): If the overall certainty in step 3 was rated down due to imprecision in either the certainty of the direct (step 1) or the indirect (step 2) estimate, and the network estimates were no longer imprecise, then we rated the certainty of evidence up by one level.
We mapped the results of the assessments for each of the above steps to a final rating, following the usual GRADE scale of: “high”, “moderate”, “low”, and “very low”. At each stage, two review authors (SM, AM) independently evaluated the certainty rating for the evidence (direct and indirect). We resolved disagreements through discussion and, where necessary, through consultation with a third review author.
When interpreting the relative effects of all COX‐I drugs, the summary of findings tables included the network effect estimates and certainty judgments for the comparisons between each of the COX‐I drugs versus placebo as the comparator. Given the potential complexity of the summary of findings tables with multiple comparisons, we created a single summary of findings table for each of the outcomes listed above, which was structured based on recent recommendations from the GRADE working group (Yepes‐Nuñez 2019). Any differences between the protocol and the final review was outlined in the “Differences between protocol and review" section.
Results
Description of studies
Results of the search
Database searches identified 7155 records; trial register searches 646; and conference websites 35. After removing 2158 duplicates, 5678 records were available for screening. We excluded 5614 records based on title/abstract; assessed 64 full‐text articles, of which 31 were excluded with reasons. We further identified three studies that are awaiting classification (Seok 1998, Akbari Asbagh 2015, Kalani 2016) and two ongoing trials on prophylactic use of acetaminophen (NCT03641209;NCT04459117), leaving 28 studies which were included in this review. The results of the search conducted in December 2021 are shown in Figure 1.
1.
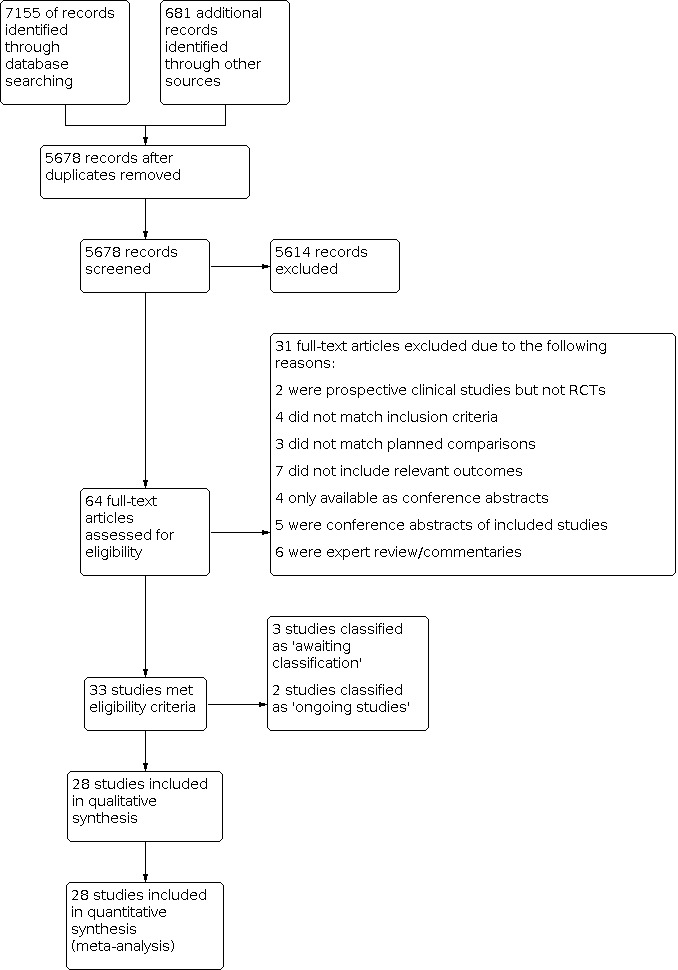
Study flow diagram.
Included studies
We included a total of 28 studies with 3999 participants. Individual study characteristics, inclusion criteria, treatment details, and outcomes can be found in the Characteristics of included studies table.
Studies using prophylactic indomethacin
Nineteen studies that enrolled 2877 infants used prophylactic indomethacin as the active intervention. The following section provides a brief description of the included studies.
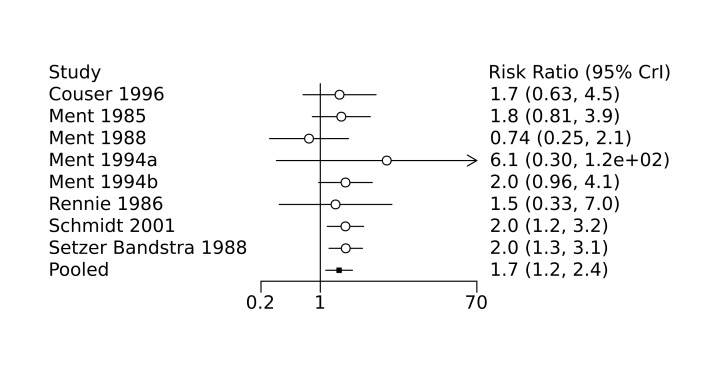
Bada 1989 conducted a single‐centre randomized controlled trial to examine the efficacy of indomethacin in preventing intraventricular haemorrhage (IVH). Infants with a birth weight less than 1500 g were randomized to receive either prophylactic indomethacin (initial dose 0.2 mg/kg intravenously at six hours of age, followed by two doses of 0.1 mg/kg at 18 hours and 30 hours of age; recruited n = 70) or placebo (recruited n = 71). Cranial ultrasounds were performed at 6, 12 and 24 hours of age, and daily thereafter until seven days of age. Perinatal characteristics were similar between the two groups, with the exception of maternal primigravida status and use of oxytocin, both of which more often observed in the placebo group. Compared to placebo, prophylactic indomethacin was associated with a decreased incidence of IVH (grades 2 to 4; 23% of infants in the indomethacin group versus 39% of infants in the control group, P = 0.03) and severe IVH with periventricular echodensities (3% in the indomethacin group versus 14% in the control group, P = 0.02).
Couser 1996 conducted a single‐centre randomized controlled trial to examine the effect of low‐dose indomethacin on the development of haemodynamically significant patent ductus arteriosus (PDA) following prophylactic surfactant administration. Preterm infants (birth weight 600 g to 1250 g) who received prophylactic surfactant in the delivery room were randomized to receive either prophylactic indomethacin (0.1 mg/kg dose every 24 hours for a total of six doses; recruited n = 43) or placebo (0.9% sodium chloride (NaCl); recruited n = 47). Perinatal characteristics were similar between the two groups. Echocardiography was performed prior to treatment, and on postnatal day seven. Presence of a moderate to large PDA was similar between the two groups at the start of treatment, and prophylactic indomethacin was associated with a significantly decreased incidence of haemodynamically significant PDA on day seven when compared to placebo (21% of infants in the indomethacin group versus 47% of infants in the placebo group, P = 0.018). Those with a residual haemodynamically significant PDA were treated with either indomethacin or surgical ligation. No other significant differences in outcomes (including bronchopulmonary dysplasia, IVH, and mortality) were observed between the two groups, nor were any adverse events observed. Couser 2000 subsequently published a 36‐month follow‐up of this study in 2000 which examined long‐term neurodevelopmental outcomes. No significant differences in mortality or neurodevelopmental outcomes were observed between the prophylactic indomethacin and placebo groups.
Hanigan 1988 conducted a single‐centre randomized controlled trial to examine the efficacy of prophylactic low‐dose indomethacin for the prevention of IVH. Preterm infants (< 34 weeks) with a birth weight < 1500 g were randomized to receive either prophylactic indomethacin (0.1 mg/kg intravenously at 12, 24, 48 and 72 hours of age; recruited n = 56) or placebo (saline; n = 55). Perinatal characteristics were similar between the two groups. Prophylactic indomethacin was associated with lower incidence of IVH (6/56 infants in the indomethacin group versus 11/55 infants in the placebo group, P = 0.174), although the incidence of severe IVH (grade 3 to 4) was not significantly different between the two groups.
Jannatdoust 2014 conducted a single‐centre randomized controlled trial to examine the effect of prophylactic indomethacin on the development of PDA and the duration of mechanical ventilation. Preterm infants (< 32 weeks gestational age) with a birth weight 800 g to 1500 g were randomized to receive either prophylactic indomethacin (initial dose 0.2mg/kg intravenously within 12 hours after birth, followed by two doses of 0.1 mg/kg at 24 and 48 hours; recruited n = 35) or no intervention (recruited n = 35). An echocardiogram was performed on day four, cranial ultrasound was performed at two weeks of age, and the type and duration of respiratory support was recorded. Perinatal characteristics were similar between the two groups. Prophylactic indomethacin was associated with a decreased incidence of large PDA (none in the indomethacin group versus 25.7% in the control group) and duration of mechanical ventilation (both invasive and non‐invasive). Prophylactic indomethacin was also associated with a decreased incidence of grade 1 IVH (22.9% indomethacin versus 8.8% control), grade 2 IVH (25.7% indomethacin versus 5.7% control), and grade 3 IVH (5.7% indomethacin versus 2.9% control), although the incidence of grade 4 IVH was similarly low between the two groups. No adverse events were reported.
Krueger 1987 conducted a single‐centre randomized controlled trial to examine the efficacy of prophylactic indomethacin in the prevention of symptomatic PDA. Preterm infants (birth weight 750 g to 1500 g) with hyaline membrane disease received either a single dose of prophylactic indomethacin (0.2 mg/kg intravenous; recruited n = 15) at 24 hours of age, or no intervention (recruited n = 17). Baseline echocardiography was performed prior to randomization and repeated on postnatal days 3, 5, and 7. Symptomatic PDA was observed less frequently in the treatment group (1/14 surviving infants in the indomethacin group versus 9/16 surviving infants in the control group, P = 0.007). Nine infants in the control group who were diagnosed with a symptomatic PDA after randomization and were subsequently treated with indomethacin, with successful closure of the ductus observed in eight infants. Perinatal characteristics were similar between the two groups. No significant differences were observed between the two groups with regards to major neonatal morbidities, including bronchopulmonary dysplasia, necrotizing enterocolitis (NEC), and IVH, nor was there a significant difference in mortality. No adverse events were observed.
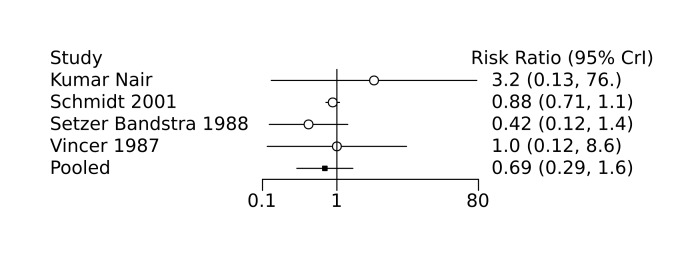
Kumar Nair 2004 conducted a single‐centre randomized controlled trial to examine the efficacy of low dose indomethacin on the development of severe IVH (grade 3 to 4). Infants greater than 26 weeks gestation with a birth weight 750 g to 1250 g were randomized to receive either prophylactic indomethacin (0.1 mg/kg/dose intravenously; recruited n = 56) or no intervention (recruited n = 59). Cranial ultrasound was performed prior to randomization and repeated on days 1, 3, and 7. When stratified by birth weight (750 g to 999 g versus 1000 g to 1250 g), prophylactic indomethacin was associated with a significantly increased incidence of severe IVH only for infants in the lower birth weight group (RR 2.05, 95% CI 1.29‐3.26, P = 0.03). In addition, for the study population as a whole, prophylactic indomethacin was also associated with a significantly increased incidence of chronic lung disease (risk ratio (RR) 1.79, 95% confidence interval (CI) 1.28 to 2.5, P = 0.005). Prophylactic indomethacin was also associated with a significantly lower incidence of PDA, but only in the higher birth weight group (P = 0.02). No significant differences in incidence of renal failure or any other neonatal outcomes were observed, including NEC, bronchopulmonary dysplasia, and mortality.
Mahony 1985 conducted a single‐centre randomized controlled trial to examine the effect of indomethacin on the development of large left‐to‐right shunting PDA. Preterm infants (birth weight 700 g to 1300 g) were randomized to receive either indomethacin (first dose 0.2 mg/kg within the first 12 to 18 hours after birth followed by two doses of 0.1 mg.kg at 12 hours and 36 hours after the first; recruited n = 51) or placebo (saline; recruited n = 53). Any infant, regardless of study arm, who developed a large left‐to‐right shunting PDA was treated with indomethacin, surgical ligation or both. Perinatal characteristics, cardiac parameters, and initial ventilator settings were similar between the two groups, with the exception of the presence of hyaline membrane disease which was observed less frequently in those treated with indomethacin (42/53 infants in the placebo group versus 36/51 infants in the indomethacin group). No significant differences were noted between the groups with regards to the primary outcomes of duration of oxygen therapy or intubation, nor was there any significant difference in days to regain birth weight or incidence of surgical ligation of the PDA. Prophylactic indomethacin was associated with a reduced incidence of large left‐to‐right shunting PDA (2/51 infants in the indomethacin group versus 11/53 infants in the placebo group, P = 0.025). No significant effect on mortality was observed, nor were any complications observed. This study was stopped early due to recruitment challenges.
Maruyama 2012 assessed intestinal and renal blood flow in a single‐centre subset of infants participating in a multi‐centre randomized controlled trial of prophylactic indomethacin for the reduction of IVH and PDA. Preterm infants participating in the larger study who had been randomized to receive either prophylactic indomethacin (0.1 mg/kg/dose intravenously for a total of three doses; n = 10) or placebo (n = 9) were examined. Baseline perinatal characteristics were similar between the two groups, with the exception of birthweight which was lower in the indomethacin group (median 677 g, range 528 g to 936 g) compared to the placebo group (median 800 g, range 692 g to 946 g) despite similar gestational ages. Flow velocity in the right renal artery and superior mesenteric artery was measured by Doppler ultrasound before and after the initial dose of indomethacin or placebo. Compared to placebo, prophylactic indomethacin was associated with significantly increased post‐dose end‐diastolic flow velocity in both the renal artery (P = 0.04) and the superior mesenteric artery (P = 0.02), but not an increase in regional vascular resistance.
Ment 1985 conducted a single‐centre randomized controlled trial to examine the efficacy of indomethacin in the prevention of IVH. Preterm infants (birth weight 600 g to 1250 g) without ultrasound evidence of IVH at six hours after birth were randomized to receive either prophylactic intravenous indomethacin (recruited n = 24) or placebo (saline; recruited n = 24). The indomethacin dosing regimen was reduced after the first 10 patients due to observed oliguria (initial dose 0.2 mg/kg followed by four doses of 0.1 mg/kg every 12 hours, reduced to 0.1 mg/kg every 12 hours for a total of five doses). Cranial ultrasounds were performed at 6, 18, 30, 42, and 54 hours after birth, and on postnatal days 4, 5, 7, 14, and 20. Perinatal characteristics and the presence of PDA on day one were similar between the two groups. Indomethacin was associated with a significant reduction in the incidence of IVH (6/24 infants in the indomethacin group versus 14/24 infants in the placebo group, P = 0.02). Treatment with indomethacin was also associated with a significant decrease in serum prostaglandin levels and an increased rate of PDA closure (84% in the indomethacin group versus 60% in the placebo group) independent of the presence of IVH.
Ment 1988 conducted a single‐centre randomized controlled trial to examine the efficacy of prophylactic low‐dose indomethacin in the prevention of IVH, and the effect on urine output. Preterm infants with a birth weight of 600 g to 1250 g were randomized to receive either prophylactic indomethacin (0.1mg/kg intravenous, first dose at 6‐12 hours of age followed by two additional doses at 24 hour intervals; recruited n = 19) or placebo (saline; recruited n = 17). Perinatal characteristics were similar between the two groups. Prophylactic indomethacin was associated with a decrease in the incidence of IVH compared to placebo (2/19 infants in the indomethacin group versus 8/17 infants in the placebo group, P = 0.02). In addition, among infants with a PDA shunting left‐to‐right prior to treatment, indomethacin was associated with higher rates of ductal closure on postnatal day five compared to placebo (64% versus 33%, respectively). In this study, indomethacin was not associated with significant oliguria, electrolyte abnormalities, laboratory evidence of renal dysfunction, or platelet abnormalities.
Ment 1994a conducted a prospective multi‐centre randomized controlled trial to examine the efficacy of low‐dose indomethacin to prevent progression of IVH in infants with early low‐grade IVH. The study was conducted in three neonatal intensive care units (NICUs) in the USA. Infants with birth weights of 600 g to 1250 g with ultrasound evidence of grade 1 IVH at 6 to 11 hours of age were randomized to receive either prophylactic indomethacin (0.1 mg/kg intravenously every 24 hours for a total of three doses; recruited n = 27) or placebo (saline; recruited n = 34). No differences in baseline perinatal characteristics were observed between the two groups. There was no significant difference in extension of the IVH with prophylactic indomethacin compared to placebo; however, indomethacin was associated with an increased incidence of PDA closure by postnatal day five when compared to control (P = 0.003). No adverse events were reported.
Ment 1994b conducted a multi‐centre prospective randomized control trial to examine the effect of low‐dose indomethacin on prevention of IVH (both incidence and severity). The study was conducted in three NICUs in the USA. Infants with birth weights 600 g to 1250 g and no ultrasound evidence of IVH at 6 to 11 hours of age were randomized to receive either prophylactic indomethacin (0.1 mg/kg intravenously every 24 hours for a total of three doses; recruited n = 209) or placebo (saline; recruited n = 222). Serial cranial ultrasounds were performed at 24 and 48 hours of age, and then on postnatal days 4, 7, 14, and 21. Echocardiography was performed on postnatal days 1, 2, 3, and 5. Baseline perinatal characteristics were similar between the two groups. Compared to placebo, prophylactic indomethacin was associated with significantly decreased incidence of IVH (12% of infants in the indomethacin group versus 18% of infants in the placebo group, P = 0.03), as well as decreased incidence of grade 4 IVH (4% of infants with IVH in the indomethacin group versus 25% of infants with IVH in the placebo group, P = 0.01). Prophylactic indomethacin was also associated with a significantly increased rate of PDA closure when compared with control (10% of infants in the indomethacin group versus 34% of infants in the placebo group, P < 0.001). No adverse events were reported. Ment 1996 subsequently conducted a 36‐month follow‐up of this study population to examine neurodevelopmental outcomes. No significant differences were observed between the two groups with regards to cerebral palsy, blindness or deafness. Stanford‐Binet IQ scores were available for 126 infants and were also similar between the two groups (89.6 [standard deviation (SD) 19.92] in the indomethacin group versus 85.0 [SD 20.79] in the placebo group). Ment 2000 conducted another follow‐up of this study that examined neurodevelopmental outcomes at 4.5 years of age. The incidence of cerebral palsy was similar to that observed at 36 months. Compared to placebo, the incidence of intellectual disability was lower among children who had received prophylactic indomethacin (IQ < 70: 9% indomethacin versus 17% placebo; IQ 70 to 80: 12% indomethacin versus 18% placebo; and IQ > 80: 79% indomethacin versus 65% placebo). Vocabulary skills were also stronger among children who had received indomethacin compared to placebo. Vohr 2003 also conducted a neurodevelopmental follow‐up of this study at school age (eight years). Children with a history of IVH were more likely to have neurodevelopmental challenges (cerebral palsy, hearing impairment, lower IQ) as well as lower daily living skills scores and greater need of educational supports. Severe IVH (grade 3 to 4), periventricular leukomalacia (PVL), and male gender were all associated with higher incidence of neurodevelopmental challenges. No effect of prophylactic indomethacin on outcomes was demonstrated. Ment 2004 conducted a further follow‐up study of this population to examine the sex‐specific effect of indomethacin on neurodevelopmental outcomes at three to eight years of age. Prophylactic indomethacin in boys was associated with a significant decrease in the incidence of both IVH and PVL, and was associated with higher verbal scores, when compared to the effects of prophylactic indomethacin in girls. Finally, Luu 2009 examined neurodevelopmental outcomes at 12 years of age in this population and found no association between prophylactic indomethacin and IQ scores.
Morales‐Suarez 1994 conducted a single‐centre randomized controlled trial to examine the effect of prophylactic low‐dose indomethacin on IVH in preterm infants on mechanical ventilation. Infants born between 28 to 36 weeks gestational age (GA) and requiring mechanical ventilation were randomized to intravenous indomethacin (three doses of 0.1 mg/kg/dose every 12 hours) (n = 40) versus placebo (n = 40). Parenteral fluids were given at rates of 70, 80 and 90 mL/kg/day on days 1, 2, and 3, respectively, to maintain a minimum urine output >1.5 mL/kg/24 hours, and urinary density between 1.005 and 1.010. Each participant was mechanically ventilated. Baseline perinatal characteristics were similar between the two groups. Compared to placebo, prophylactic indomethacin was associated with significantly decreased incidence of both grade 3 IVH (4/40 in indomethacin group versus 8/40 in the placebo group;P < 0.005) and grade 4 IVH (2/40 in indomethacin group versus 5/40 in the placebo group; P < 0.005).
Rennie 1986 conducted a single‐centre randomized controlled trial to examine the effects of indomethacin in preterm infants. Preterm infants (birth weight <1750g) less than 24 hours of age and without ultrasound evidence of IVH at the time of enrolment were randomized to receive either indomethacin (three doses of 0.2mg/kg at 24‐hour intervals; recruited n = 24) or placebo (saline; recruited n = 26). Cranial ultrasounds were performed daily for the first four days, followed by weekly scans thereafter. Infants in the placebo group were more likely to be male and had lower 1‐minute Apgar scores. The incidence of left‐to‐right shunting PDA requiring treatment was significantly lower in those who received prophylactic indomethacin (1/24 infants in the indomethacin group versus 8/26 infants in the placebo group, P = 0.03). The incidence of gastrointestinal bleeding was significantly higher in those who received prophylactic indomethacin (7/24 infants in the indomethacin group versus 0/26 infants in the placebo group, P = 0.01). No significant differences were observed between the two groups with regard to the duration of mechanical ventilation or oxygen requirement, nor were any significant differences observed in the incidence of renal impairment, IVH, or mortality.
Schmidt 2001 conducted a multi‐centre randomized controlled trial to examine the effect of prophylactic low‐dose indomethacin on survival without neurosensory impairment. The study was conducted at 32 neonatal intensive care units in Canada, Australia, New Zealand, Hong Kong, and the USA. Preterm infants with a birth weight 500 g to 999 g were randomized to receive either prophylactic indomethacin (0.1 mg/kg intravenously once daily for three days; recruited n = 574) or placebo (saline; recruited n = 569). Baseline perinatal characteristics were similar between the two groups. The incidence of the composite outcome of death or significant neurosensory impairment (including cerebral palsy, cognitive delay, deafness or blindness) at 18 months of age was not significantly different between the two groups (P = 0.61). However, prophylactic indomethacin was associated with a decreased incidence of PDA (P < 0.001) and severe IVH (P = 0.02). No differences were observed between the two groups with regards to other major neonatal morbidities (including chronic lung disease, NEC, and retinopathy of prematurity) or other neurologic morbidities (including seizures, severe hydrocephalus, and microcephaly). Ohlsson 2005 subsequently conducted a secondary analysis of this study which examined whether prophylactic indomethacin had a sex‐mediated effect on short‐ and long‐term neurodevelopmental outcomes. Compared to placebo, prophylactic indomethacin reduced the incidence of the composite outcome (as described above) more for girls compared to boys (\P = 0.048).No significant sex‐mediated effect on any of the other short‐ or long‐term neurodevelopmental outcomes were observed. Schmidt 2006 also conducted an additional analysis of this study to examine the effect of prophylactic indomethacin on the development of bronchopulmonary dysplasia among infants with and without PDA. Among infants with PDA, prophylactic indomethacin was not associated with bronchopulmonary dysplasia. In contrast, among infants without PDA, prophylactic indomethacin was associated with a significantly increased incidence of bronchopulmonary dysplasia (43% of infants in the indomethacin group versus 30% of infants in the placebo group, P = 0.015). In addition, Zupancic 2006 conducted an economic analysis of this study to examine the cost‐effectiveness of indomethacin prophylaxis for PDA prevention, which was not able to demonstrate an economic benefit.
Setzer Bandstra 1988 conducted a single‐centre randomized controlled trial to examine the efficacy of prophylactic indomethacin compared to placebo for the prevention of both IVH and PDA. Preterm infants (birth weight< 1300 g) requiring oxygen who did not have an IVH grade 2 or higher (assessed by pre‐study cranial ultrasound) were randomized to receive either prophylactic intravenous indomethacin (initial dose 0.2 mg/kg within 12 hours of birth, followed by two doses of 0.1 mg/kg at intervals of 12 hours; recruited n = 99) or placebo (0.45% NaCl; recruited n = 100). Perinatal characteristics were similar between the two groups, although there was a greater number of female infants in the placebo group compared to the indomethacin group (57% versus 48%, respectively). Prophylactic indomethacin was associated with a significant decrease in the incidence of IVH grades 2 to 4 compared to placebo (23% versus 46%, P < 0.002). Prophylactic indomethacin was also associated with a significant decrease in the incidence of clinically significant PDA compared to placebo (11% versus 42%, P < 0.001). Compared to the placebo group, prophylactic indomethacin was associated with oliguria (P < 0.001), but no significant differences were noted between the two groups with regard to duration of oxygen therapy or mechanical ventilation, duration of hospitalization, or any of the major neonatal outcomes including NEC, chronic lung disease, sepsis, retinopathy of prematurity, and mortality. The study abstract was published in 1984 as a conference proceeding that showed preliminary results identical to those described above (Setzer 1984a). A second conference abstract was also published in 1984 which demonstrated that prophylactic indomethacin was associated with decreased platelet count and prolonged bleeding time in the first postnatal week, although no data on adverse outcomes related to these laboratory abnormalities were presented (Setzer 1984b).
Supapannachart 1999 conducted a single‐centre randomized controlled trial to examine the efficacy of prophylactic indomethacin to prevent the development of symptomatic PDA. Preterm infants with a birth weight less than 1250 g were randomized to receive either prophylactic indomethacin (initial dose 0.2 mg/kg intravenous within the first 24 hours after birth, followed by two doses of 0.1 mg/kg at 12 hours intervals; recruited n = 15) or placebo (recruited n = 15). Perinatal characteristics were similar between the two groups, with the exception of surfactant administration which occurred more frequently in the indomethacin group. Prophylactic indomethacin was associated with a significantly decreased incidence of symptomatic PDA compared to placebo (4/15 infants in the indomethacin group versus 12/15 infants in the placebo group, P < 0.005). No significant differences in major neonatal morbidities were observed, nor was there any significant difference in mortality. No adverse respiratory, renal or haematologcal effects were observed.
Vincer 1987 conducted a single‐centre randomized controlled trial to examine the effect of prophylactic indomethacin on the development of chronic pulmonary insufficiency of prematurity. Infants with a birth weight less than 1500 g who required respiratory support (invasive or non‐invasive positive pressure ventilation) at 12 hours of age were randomized to receive either indomethacin (three doses of 0.2 mg/kg intravenously at 12, 24, and 36 hours of age; recruited n = 15) or placebo (saline; recruited n = 15). Perinatal characteristics and baseline respiratory support parameters were similar between the two groups. Among infants who required invasive positive pressure ventilation, placebo was associated with earlier successful weaning of respiratory support compared to indomethacin (P < 0.05), although oxygen requirement was not significantly different. Infants who received indomethacin were less likely to have symptomatic PDA (1/15 infants in the indomethacin group versus 5/15 infants in the placebo group, P < 0.10). Indomethacin was also associated with hyponatraemia and less weight loss in the first 7 postnatal days compared to placebo. No significant differences were observed between the two groups with regards to the incidence of IVH, NEC, or mortality, and no adverse events were observed. Vincer 1998 subsequently conducted a 2‐year follow‐up of this study which examined the incidence of cerebral palsy in those treated with prophylactic indomethacin. Of those infants assessed at two years, prophylactic indomethacin was associated with an increased incidence of cerebral palsy (5/12 in the indomethacin group versus 1/12 in the control group, P = 0.15), although it was not associated with an increase in the incidence of severe IVH or cystic periventricular leukomalacia.
Vogtmann 1988 conducted a single‐centre randomized controlled trial to examine the effect of prophylactic oral indomethacin in preterm infants. Infants with a birthweight of ≤ 1500 g and GA ≤ 30 weeks were randomized to oral indomethacin at a dose 0.2 mg/kg/day from days three to five (n = 19) or standard of care (n = 22). There was no statistically significant difference in any clinically relevant outcomes such as mortality or NEC between the two groups.
Studies using prophylactic ibuprofen
Seven studies that enrolled 914 infants used prophylactic ibuprofen as the active intervention. The following section provides a brief description of the included studies.
Dani 2000 conducted a two‐centre randomized controlled trial to assess the efficacy of prophylactic ibuprofen for reducing the occurrence of PDA. Preterm infants (< 34 weeks’ gestational age) with respiratory distress syndrome were randomly assigned to receive intravenous ibuprofen (initial dose 10 mg/kg, followed by 5 mg/kg doses at 24 and 48 hours) either prophylactically within the first 24 hours of life (n = 40), or after diagnosis of a PDA by echocardiography (n = 40). Oxygenation Index and Ventilatory Index (initial and highest) were used to measure severity of respiratory distress syndrome (RDS). which were similar between the two groups. Both modes of treatment were found to be effective in closing the PDA. However, early prophylactic treatment significantly reduced the occurrence of PDA on day three of life (prophylaxis 3/40 infants versus post‐echocardiography 21/40 infants, P < 0.0001). There were no significant differences between the two groups in the frequency of bronchopulmonary dysplasia, IVH, NEC, or retinopathy of prematurity.
Dani 2005 conducted a multi‐centre randomized controlled trial to compare the efficacy of prophylactic ibuprofen versus placebo to reduce the occurrence of IVH, as well as the progression of low‐grade (none or grade 1) IVH to higher grade (grades 2 to 4) IVH. The study was conducted at seven Italian NICUs. Preterm infants (< 28 weeks’ gestational age) were randomly assigned within the first six hours of life to receive either intravenous ibuprofen (initial dose 10 mg/kg, followed by 5 mg/kg doses at 24 and 48 hours; n = 77) or placebo (n = 78). Serial cranial ultrasounds and echocardiography were subsequently performed. Perinatal characteristics were similar between the two groups with the exception of gestational age at birth (ibuprofen 25.3 + 1.2 days versus placebo 25.9 + 1.1 days). The prevalence of grade 1 IVH on initial cranial ultrasound was also similar between the groups. Prophylactic ibuprofen administration did not significantly decrease the occurrence of IVH (all grades), nor was it effective in preventing progression from low‐ to higher‐grade IVH. Prophylactic administration of ibuprofen was associated with a decreased occurrence of PDA on day three of life (ibuprofen 7/77 infants versus placebo 23/78 infants, P < 0.002) No significant differences were observed between the two groups with regards to the frequency of bronchopulmonary dysplasia, NEC, retinopathy of prematurity, sepsis, or mortality.
De Carolis 2000 conducted a single‐centre randomized controlled trial to compare the efficacy of prophylactic ibuprofen versus no intervention to reduce the occurrence of PDA. Preterm infants ( <31 weeks’ gestational age) were randomized at two hours of life to receive either intravenous ibuprofen (initial dose 10 mg/kg, followed by 5 mg/kg doses at 24 and 48 hours; n = 23) or no treatment (n = 23). Perinatal characteristics and initial respiratory status were similar between the two groups. The rate of PDA closure at three days of age was significantly higher in the group that received prophylactic ibuprofen compared to the control group (P < 0.01). There were no differences between the groups with regard to mortality, IVH, NEC, and renal or haematological complications.
Gournay 2004 conducted a multi‐centre randomized controlled trial to compare the efficacy of prophylactic ibuprofen versus placebo to reduce the occurrence of PDA requiring surgical intervention. The study was conducted at 11 NICUs in France. Preterm infants (< 28 weeks’ gestational age) were randomized within the first six hours of life to receive either intravenous ibuprofen (initial dose 10 mg/kg, followed by 5 mg/kg doses at 24 and 48 hours; n = 65) or placebo (saline; n = 66). Recruitment stopped early (135/250 patients recruited) due to concerns regarding development of severe pulmonary hypertension in three infants in the prophylactic ibuprofen group. No difference in mortality was noted between the two groups; however, compared to placebo, ibuprofen prophylaxis did reduce the need for surgical ligation of the PDA (P = 0.03).
Kanmaz 2013 conducted a single‐centre randomized controlled trial to compare the efficacy of prophylactic oral ibuprofen versus no intervention for the prevention of a haemodynamically significant PDA. Preterm infants (< 28 weeks’ gestational age) weighing < 1000 g were randomly assigned to either oral ibuprofen (initial dose 10 mg/kg, followed by 5 mg/kg doses at 24 and 48 hours; recruited n = 23) or no intervention (recruited n = 23). The study was terminated early due to adverse events in the prophylactic ibuprofen group, which included two infants with gastrointestinal bleeding, two infants with spontaneous intestinal perforation, and two infants with renal failure. Of those infants who completed the study, the rate of haemodynamically significant PDA was reduced by was not significantly different between the two groups.
Sangtawesin 2006 conducted a single‐centre randomized controlled trial to compare efficacy of prophylactic oral ibuprofen versus placebo for the prevention of symptomatic PDA. Preterm infants (28 to 32 weeks’ gestational age) with birth weight < 1500 g were randomly assigned to either oral ibuprofen (three doses of 10 mg/kg, first dose administered within the first 24 hours of life and then at 24 and 48 hours thereafter; n = 22) or placebo (oral starch suspension; n = 20). Perinatal characteristics and the presence of asymptomatic PDA at the time of first dose administration were similar between the two groups. Compared to placebo, prophylactic treatment with ibuprofen was associated with reduced presence of symptomatic PDA on postnatal day three (ibuprofen 0/22 infants versus placebo 5/20 infants, P = 0.015) and postnatal day 7 (ibuprofen 0/22 infants versus placebo 6/20 infants, P = 0.006), respectively. No significant differences were noted between the groups for the rate of pulmonary hypertension, bronchopulmonary dysplasia, IVH, NEC, or retinopathy of prematurity. A slightly higher, non‐significant risk of gastrointestinal bleeding was noted in the prophylactic ibuprofen group compared to the control.
Van Overmeire 2004 conducted a multi‐centre randomized controlled trial to compare the efficacy of prophylactic ibuprofen versus placebo to reduce the occurrence of PDA and IVH. The study was conducted at seven NICUs in Belgium. Preterm infants (< 31 weeks’ gestational age) were randomized within the first six hours of birth to receive either intravenous ibuprofen (initial dose 10 mg/kg, followed by 5 mg/kg doses at 24 and 48 hours; n = 205) or placebo (saline; n = 210). No statistically significant difference was observed for rates of IVH between the two groups (RR 0.97 [95% CI 0.51,1,82]). However, rates of PDA closure on day three were higher in the prophylactic ibuprofen group compared to the control group (RR 1.40 [1.23 to 1.59]). No significant differences in other clinical outcomes, including NEC, bronchopulmonary dysplasia, and mortality, or serious adverse events were observed. The study abstract was published in 2002 as a conference proceeding that showed results identical to those described above (Van Overmeire 2002).
Studies using prophylactic acetaminophen
Two studies that enrolled 208 infants used prophylactic acetaminophen as the active intervention. The following section provides a brief description of the included studies.
Bagheri 2018 conducted a single‐centre randomized controlled trial to compare the efficacy of prophylactic acetaminophen versus non‐intervention in the prevention of PDA. Preterm infants (< 34 weeks’ gestational age) were randomly assigned to receive either intravenous acetaminophen (initial dose 20 mg/kg followed by 7.5 mg/kg doses every six hours for the first three postnatal days; recruited n = 80) or no intervention (recruited n = 80). An echocardiogram was performed on postnatal day four. Perinatal characteristics were similar between the two groups. Compared to no intervention, prophylactic acetaminophen was associated with a significantly lower incidence of PDA (12/80 in the treatment group compared to 57/80 in the control group, P < 0.001). Mean ventilator time, mean cardiac shortening fraction, and mortality were not significantly different between the two groups. No adverse events were observed.
Harkin 2016 conducted a randomized controlled trial to compare the effect of prophylactic acetaminophen versus placebo on the closure of the ductus arteriosus. Preterm infants (< 32 weeks gestational age) were randomly assigned to receive either intravenous acetaminophen (initial dose 20 mg/kg, given within 24 hours of birth, followed by 7.5 mg/kg every six hours for a total of four days; recruited n = 23) or placebo (0.45% NaCl; recruited n = 25). An echocardiogram was performed prior to the first dose and repeated daily until day five. Perinatal characteristics were similar between the two groups, as were echocardiographic measurements of the ductus arteriosus prior to the first dose. Prophylactic acetaminophen was associated with earlier closure of the ductus arteriosus (P = 0.045). Serum acetaminophen levels were noted to be within the therapeutic range, and no short‐term adverse effects were observed. Juujärvi 2019 subsequently conducted a two‐year follow‐up of this study which examined the long‐term safety and outcomes associated with prophylactic acetaminophen. Forty‐four of the 48 infants originally recruited (92%) were assessed using a parental questionnaire in conjunction with clinical and neurodevelopmental assessments. No long‐term adverse cardiac outcomes were observed, and neurodevelopmental outcomes were similar between the two groups.
Excluded studies
We excluded 31 publications for the following reasons.
1. Two publications (Liebowitz 2017, Varvarigou 1996) were excluded as they were not randomized controlled trials.
2. Four publications (Cotts 2009, Hammerman 1986, Kääpä 1985, Mahony 1982) were excluded because the study population did not match our inclusion criteria, which stipulated that intervention must be delivered within the first 72 hours after birth and there must be no documented clinical or echocardiographic evidence of PDA.
3. Three publications (Rubaltelli 1998, Schmidt 2011; Valls‐i‐Soler 1999) were excluded as the one of the trial interventions in each of these studies did not include any of the four interventions defined in our review (prophylactic indomethacin, prophylactic ibuprofen, prophylactic acetaminophen, placebo/no treatment).
4. Seven publications were excluded (Alfaleh 2008, Gregoire 2004, Harma 2018, Ment 1999, Naulaers 2005, Pleacher 2004, Vohr 1999) as they did not include any of our pre‐defined clinical outcomes.
5. Four publications (Domanico 1994, Gutierrez 1987, Puckett 1985, Zarkesh 2013) were excluded as they were available as conference abstracts only, and hence, we were unable to assess the quality of the study methodology.
6. Five publications (Meau‐Petit 2005, Ment 1987, Morales‐Suarez 1992, Roze 2003, van Overmeire 2002) were excluded as they are conference abstracts of studies already included in our review.
7. Six publications (Barrington 1986, Hammerman 2005, McGuire 2002, Ment 1998, Schmidt 2002, Tyson 2002) were excluded as they were either expert reviews or commentaries.
For further details see Characteristics of excluded studies
Risk of bias in included studies
For the summary of the authors' judgements on the risk of bias in individual studies, please see Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both randomization and allocation procedures were clearly described in seven studies (Harkin 2016; Jannatdoust 2014; Kumar Nair 2004; Ment 1994b; Schmidt 2001; Setzer Bandstra 1988; Van Overmeire 2004). One or both of randomization procedure and allocation concealment was judged to have unclear risk of bias in the remaining 21 studies. No study was judged to have a high risk of selection bias.
Blinding
Blinding processes were clearly described in 18 studies (Bada 1989; Setzer Bandstra 1988; Couser 1996; Dani 2005; Gournay 2004; Hanigan 1988; Harkin 2016; Mahony 1985; Ment 1985; Ment 1988; Ment 1994a; Ment 1994b; Rennie 1986; Sangtawesin 2006; Schmidt 2001; Supapannachart 1999; Van Overmeire 2004; Vincer 1987), while eight studies (Bagheri 2018; Dani 2000; De Carolis 2000; Jannatdoust 2014; Kanmaz 2013; Krueger 1987; Kumar Nair 2004; Vogtmann 1988) were judged to be at a high risk of bias for either performance or detection bias.
Incomplete outcome data
Only one study was judged to be at a high risk for attrition bias as infants who died prior to day eight were removed from the study (Vogtmann 1988). We judged all the remaining studies to be at low risk for attrition bias.
Selective reporting
Only three studies had a study protocol registered a priori for us to be able to judge the domain of selective outcome reporting. Out of these three studies, two (Harkin 2016; Kanmaz 2013) were at low risk for selective outcome reporting while one (Maruyama 2012) was judged to be at a high risk for selective outcome reporting. We were unable to judge the reporting bias for the remaining studies due to lack of an a priori published protocol available for comparison.
Other potential sources of bias
No studies were judged to be at a high risk for other potential sources of bias.
Effects of interventions
See: Table 1
Out of the 13 a priori defined outcome measures, outcome data on more than one COX‐I drug were available for 11 outcomes. Therefore, effects of interventions have been summarized for 11 out of the 13 listed outcomes where a network meta‐analysis was possible. Further, none of the pre‐defined subgroup analyses (based on gestational age, birth weight or timing of initiation of prophylaxis) were possible due to lack of complete data in either subgroup in each category. Instead, we performed a post‐hoc sensitivity analysis of studies that specifically reported on infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). We reported the sensitivity analysis results for those clinically relevant outcomes where subgroup analyses were planned a priori. The effects of the interventions as obtained on statistical analysis using Bayesian random‐effects model were as follows (see Table 1).
Primary outcomes
Severe intraventricular haemorrhage (IVH) (grade 3 or 4)
Twenty‐three studies (n = 3540) reported on this outcome [Indomethacin versus placebo (16 studies, 2629 infants); ibuprofen versus placebo (6 studies, 863 infants) and acetaminophen versus placebo (1 study, 48 infants)]. The network diagram is presented in Figure 4. Each node in the network diagram indicates a treatment modality and is sized proportionally to the number of participants who received the treatment modality. Each line connecting two nodes indicates a direct comparison between two modalities, and the thickness of each is proportional to the number of studies directly comparing the two modalities.
4.
Network plot for severe intraventricular hemorrhage
Initial pairwise meta‐analysis using Bayesian random‐effects model showed a statistically significant reduction in severe IVH with indomethacin compared to placebo (16 studies, 2629 infants; risk ratio (RR) 0.60, 95% credible interval (CrI) 0.45 to 0.80) (Figure 5). No statistically significant difference was observed with ibuprofen versus placebo (6 studies, 863 infants; RR 0.57, 95% CrI 0.26 to 1.3) (Figure 6) or with acetaminophen versus placebo (1 study, 48 infants; RR 1.09, 95% CrIs 0.07 to 17.64).
5.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for severe intraventricular hemorrhage.
A RR<1 favors the intervention. CrI, Credible intervals
6.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for severe intraventricular hemorrhage
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed that indomethacin significantly reduced severe IVH compared to placebo (Network RR 0.66, 95% CrIs 0.49, 0.87; moderate certainty). No such effects were observed with ibuprofen (Network RR 0.69, 95% CrIs 0.41, 1.1; moderate certainty) or acetaminophen (Network RR 1.2, 95% CrIs 0.04, 55.0; very low certainty) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 7; Table 2. Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 8). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Both indomethacin (median rank 2, 95% CrI 1 to 3) and ibuprofen (median rank 2, 95% CrI 1 to 4) ranked similarly for reduction of severe IVH (Figure 9). Based on the mean surface under the cumulative ranking curve (SUCRA) values, indomethacin had the highest SUCRA (0.74) followed by ibuprofen (0.67).
7.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for severe intraventricular hemorrhage
A RR<1 favors the intervention. CrI, Credible intervals
1. Network effect estimates and ranking statistics for severe intraventricular hemorrhage (grade 3 or 4).
|
Acetaminophen Mean SUCRA, 0.39; median rank, 4 (95% CrI, 1‐4) |
|||
| 1.69 (0.05, 85.3) |
Ibuprofen Mean SUCRA, 0.67; median rank, 2 (95% CrI, 1‐4) |
||
| 1.76 (0.06, 82.9) | 1.05 (0.59, 1.86) |
Indomethacin Mean SUCRA, 0.74; median rank, 2 (95% CrI, 1‐3) |
|
| 1.17 (0.04, 55.2) | 0.69 (0.41, 1.14) | 0.66 (0.49, 0.87) |
Placebo Mean SUCRA, 0.20; median rank, 3 (95% CrI, 2‐4) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
8.
Comparison‐adjusted funnel plot for severe intraventricular hemorrhage
9.
Ranking probability (rankogram) of each treatment modality for severe intraventricular hemorrhage
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Sensitivity analysis
We conducted a sensitivity analysis of studies that specifically reported on infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). 5 studies (n = 1335) that compared indomethacin versus placebo and 3 studies (n = 332) that compared ibuprofen versus placebo reported on severe IVH in infants in this specific gestational age and/or birth weight. Bayesian random‐effects network meta‐analysis showed no statistically significant difference between indomethacin versus placebo (Network RR 0.81, 95% CrIs 0.37, 2.0) as well as ibuprofen versus placebo (Network RR 0.46, 95% CrIs 0.14, 1.2) for the outcome of severe IVH. Ibuprofen (median rank 1, 95% CrI 1 to 3; mean SUCRA, 0.91) ranked as the best treatment for reduction of severe IVH followed by indomethacin (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.43) and placebo (median rank 2, 95% CrI 1 to 3; mean SUCRA), 0.16) in this specific gestational age and/or birth weight group.
Mortality (at discharge or at last reported follow‐up, whichever is later)
Twenty‐eight studies (n = 3999) reported on this outcome [Indomethacin versus placebo (19 studies, 2877 infants); ibuprofen versus placebo (7 studies, 914 infants) and acetaminophen versus placebo (2 studies, 208 infants)]. The network diagram is presented in Figure 10.
10.
Network plot for mortality
Initial pairwise meta‐analysis using Bayesian random‐effects model showed no statistically significant differences in mortality with indomethacin compared to placebo (19 studies, 2877 infants; RR 0.82, 95% CrI 0.63 to 1.1) (Figure 11), ibuprofen versus placebo (7 studies, 914 infants; RR 0.83, 95% CrI 0.55 to 1.3) (Figure 12), or with acetaminophen versus placebo (2 studies, 208 infants; RR 0.43, 95% CrI 0.11 to 1.8) (Figure 13).
11.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for mortality
A RR<1 favors the intervention. CrI, Credible intervals
12.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for mortality
A RR<1 favors the intervention. CrI, Credible intervals
13.
Forest plot of pairwise meta‐analysis between acetaminophen and placebo (conducted using Bayesian random‐effects model) for mortality
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed no statistically significant reduction in mortality with indomethacin (Network RR 0.85, 95% CrIs 0.64, 1.05; moderate‐certainty), ibuprofen (Network RR 0.83, 95% CrIs 0.57, 1.18; low‐certainty) or acetaminophen (Network 0.49, 95% CrIs 0.16, 1.36; very low‐certainty) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 14; Table 3. Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 15). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Acetaminophen (median rank 1, 95% CrI 1 to 4) ranked as the best treatment for reduction in mortality followed by ibuprofen (median rank 2, 95% CrI 1 to 4) and indomethacin (median rank 2, 95% CrI 1 to 4) (Figure 16). Based on the mean SUCRA values, acetaminophen had the highest SUCRA (0.87).
14.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for mortality
A RR<1 favors the intervention. CrI, Credible intervals
2. Network effect estimates and ranking statistics for mortality.
|
Acetaminophen Mean SUCRA, 0.87; median rank, 1 (95% CrI, 1‐4) |
|||
| 0.58 ( 0.19, 1.76) |
Ibuprofen Mean SUCRA, 0.51; median rank, 2 (95% CrI, 1‐4) |
||
| 0.58 ( 0.19, 1.69) | 0.99 ( 0.66, 1.53) |
Indomethacin Mean SUCRA, 0.52; median rank, 2 (95% CrI, 1‐4) |
|
| 0.49 ( 0.16, 1.36) | 0.83 ( 0.57, 1.18) | 0.85 ( 0.64, 1.05) |
Placebo Mean SUCRA, 0.095; median rank, 4 (95% CrI, 3‐4) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
15.
Comparison‐adjusted funnel plot for mortality
16.
Ranking probability (rankogram) of each treatment modality for mortality
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Sensitivity analysis
We conducted a sensitivity analysis of studies that specifically reported on infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). 6 studies (n = 1421) that compared indomethacin versus placebo and 3 studies (n = 332) that compared ibuprofen versus placebo reported on mortality in infants in this specific gestational age and/or birth weight. Bayesian random effects network meta‐analysis showed no statistically significant difference between indomethacin versus placebo (Network RR 1.2, 95% CrIs 0.74, 1.9) as well as ibuprofen versus placebo (Network RR 0.78, 95% CrIs 0.42, 1.4) for the outcome of mortality. Ibuprofen (median rank 1, 95% CrI 1 to 3; mean SUCRA, 0.87) ranked as the best treatment for reduction in mortality followed by placebo (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.48) and indomethacin (median rank 3, 95% CrI 1 to 3; mean SUCRA, 0.15) in this specific gestational age and/or birth weight group.
Secondary outcomes
Receipt of pharmacotherapy for symptomatic patent ductus arteriosus (PDA)
Twenty‐two studies (n = 3240) reported on this outcome [Indomethacin versus placebo (13 studies, 2117 infants); ibuprofen versus placebo (7 studies, 915 infants) and acetaminophen versus placebo (2 studies, 208 infants)]. The network diagram is presented in Figure 17.
17.
Network plot for pharmacotherapy for symptomatic PDA
Initial pairwise meta‐analysis using Bayesian random‐effects model showed a statistically significant reduction in treatment for symptomatic PDA with indomethacin versus placebo (13 studies, 2117 infants; RR 0.30, 95% CrI 0.19 to 0.47) (Figure 18) and ibuprofen versus placebo (7 studies, 915 infants; RR 0.18, 95% CrI 0.08 to 0.41) (Figure 19). No statistically significant difference in treatment for symptomatic PDA was noted with acetaminophen versus placebo (2 studies, 208 infants; RR 0.39, 95% CrI 0.08 to 1.8) (Figure 20).
18.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for pharmacotherapy for symptomatic PDA
A RR<1 favors the intervention. CrI, Credible intervals
19.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for pharmacotherapy for symptomatic PDA
A RR<1 favors the intervention. CrI, Credible intervals
20.
Forest plot of pairwise meta‐analysis between acetaminophen and placebo (conducted using Bayesian random‐effects model) for pharmacotherapy for symptomatic PDA
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed a statistically significant reduction in treatment for symptomatic PDA with both indomethacin (Network RR 0.30, 95% CrIs 0.17, 0.43) as well as ibuprofen (Network RR 0.20, 95% CrIs 0.098, 0.33) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 21; Table 4. No statistically significant difference in treatment for symptomatic PDA was noted with acetaminophen versus placebo (Network RR 0.32, 95% CrIs 0.13, 1.1) (Figure 21). Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 22). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Ibuprofen (median rank 1, 95% CrI 1 to 3) ranked as the best treatment for reduction in need for PDA pharmacotherapy followed by indomethacin (median rank 2, 95% CrI 1 to 3) (Figure 23). Based on the mean SUCRA values, ibuprofen had the highest SUCRA (0.90).
21.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for pharmacotherapy for symptomatic PDA
3. Network effect estimates and ranking statistics for receipt of pharmacotherapy for symptomatic PDA.
|
Acetaminophen Mean SUCRA, 0.52; median rank, 3 (95% CrI, 1‐4) |
|||
| 1.66 ( 0.57, 7.10) |
Ibuprofen Mean SUCRA, 0.90; median rank, 1 (95% CrI, 1‐3) |
||
| 1.10 ( 0.40, 4.53) | 0.66 ( 0.32, 1.43) |
Indomethacin Mean SUCRA, 0.56; median rank, 2 (95% CrI, 1‐3) |
|
| 0.32 ( 0.13, 1.12) | 0.20 ( 0.098, 0.33) | 0.30 ( 0.17, 0.43) |
Placebo Mean SUCRA, 0.01; median rank, 4 (95% CrI, 3‐4) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
22.
Comparison‐adjusted funnel plot for pharmacotherapy for symptomatic PDA
23.
Ranking probability (rankogram) of each treatment modality for pharmacotherapy for symptomatic PDA
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Surgical or interventional patent ductus arteriosus (PDA) closure
Seventeen studies (n = 2673) reported on this outcome [Indomethacin versus placebo (11 studies, 1800 infants); ibuprofen versus placebo (6 studies, 873 infants). All studies used surgical PDA closure as the intervention. The network diagram is presented in Figure 24.
24.
Network plot for surgical PDA closure
Initial pairwise meta‐analysis using Bayesian random‐effects model showed a statistically significant reduction in surgical PDA ligation with indomethacin versus placebo (11 studies, 1800 infants; RR 0.37, 95% CrI 0.18 to 0.77) (Figure 25) and ibuprofen versus placebo (6 studies, 873 infants; RR 0.17, 95% CrI 0.03 to 0.94) (Figure 26).
25.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for surgical PDA closure
A RR<1 favors the intervention. CrI, Credible intervals
26.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for surgical PDA closure
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed a statistically significant reduction in surgical PDA ligation with both indomethacin (Network RR 0.40, 95% CrIs 0.14, 0.66; moderate‐certainty) as well as ibuprofen (Network RR 0.24, 95% CrIs 0.06, 0.64; moderate‐certainty) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 27; Table 5. Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 28). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Ibuprofen (median rank 1, 95% CrI 1 to 2) ranked as the best treatment for reduction in surgical PDA ligation followed by indomethacin (median rank 2, 95% CrI 1 to 2) (Figure 29). Based on the mean SUCRA values, ibuprofen had the highest SUCRA (0.88).
27.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for surgical PDA closure
A RR<1 favors the intervention. CrI, Credible intervals
4. Network effect estimates and ranking statistics for surgical or interventional PDA closure.
|
Ibuprofen Mean SUCRA, 0.88; median rank, 1 (95% CrI, 1‐2) |
||
| 0.64 ( 0.17, 2.39) |
Indomethacin Mean SUCRA, 0.61; median rank, 2 (95% CrI, 1‐2) |
|
| 0.24 ( 0.06, 0.64) | 0.40 ( 0.14, 0.66) |
Placebo Mean SUCRA, 0.002; median rank, 3 (95% CrI, 3‐3) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
28.
Comparison‐adjusted funnel plot for surgical PDA closure
29.
Ranking probability (rankogram) of each treatment modality for surgical PDA closure
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Sensitivity analysis
We conducted a sensitivity analysis of studies that specifically reported on infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). 3 studies (n = 1287) that compared indomethacin versus placebo and 3 studies (n = 332) that compared ibuprofen versus placebo reported on surgical PDA closure in infants in this specific gestational age and/or birth weight. Bayesian random‐effects network meta‐analysis showed a statistically significant reduction in surgical PDA closure with ibuprofen versus placebo (Network RR 0.07, 95% CrIs 0.001, 0.73) but not with indomethacin versus placebo (Network RR 0.56, 95% CrIs 0.13, 3.0). Ibuprofen (median rank 1, 95% CrI 1 to 2; mean SUCRA, 0.97) ranked as the best treatment for reduction in surgical PDA ligation followed by indomethacin (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.45) and placebo (median rank 3, 95% CrI 2 to 3; mean SUCRA, 0.08) in this specific gestational age and/or birth weight group.
Necrotizing enterocolitis (NEC) (stage 2 or greater)
Twenty‐two studies (n = 3496) reported on this outcome [Indomethacin versus placebo (14 studies, 2543 infants); ibuprofen versus placebo (7 studies, 905 infants) and acetaminophen versus placebo (1 study, 48 infants)]. The acetaminophen node had zero events for NEC and therefore was removed from the network meta‐analysis as no continuity correction was applied. The network diagram is presented in Figure 30.
30.
Network plot for necrotizing enterocolitis
Initial pairwise meta‐analysis using Bayesian random‐effects model showed no statistically significant differences in NEC with indomethacin compared to placebo (14 studies, 2543 infants; RR 0.78, 95% CrI 0.45 to 1.4) (Figure 31) or ibuprofen versus placebo (7 studies, 905 infants; RR 0.63, 95% CrI 0.24 to 1.7) (Figure 32).
31.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for necrotizing enterocolitis
A RR<1 favors the intervention. CrI, Credible intervals
32.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for necrotizing enterocolitis
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random effects network meta‐analysis showed no statistically significant reduction in NEC with indomethacin (Network RR 0.76, 95% CrIs 0.35, 1.2; high‐certainty) or ibuprofen (Network RR 0.73, 95% CrIs 0.31, 1.4; high‐certainty) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 33, Table 6. Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 34). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the COX‐I drugs. Ibuprofen (median rank 1, 95% CrI 1 to 3) ranked as the best treatment for reduction in NEC followed by indomethacin (median rank 2, 95% CrI 1 to 3) (Figure 35). Based on the mean SUCRA values, ibuprofen had the highest SUCRA (0.69).
33.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for necrotizing enterocolitis
A RR<1 favors the intervention. CrI, Credible intervals
5. Network effect estimates and ranking statistics for necrotizing enterocolitis.
|
Ibuprofen Mean SUCRA, 0.69; median rank, 1 (95% CrI, 1‐3) |
||
| 0.96 ( 0.40, 2.55) |
Indomethacin Mean SUCRA, 0.66; median rank, 2 (95% CrI, 1‐3) |
|
| 0.73 (0.31, 1.4) | 0.76 (0.35, 1.2) |
Placebo Mean SUCRA, 0.15; median rank, 3 (95% CrI, 2‐3) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
34.
Comparison‐adjusted funnel plot for necrotizing enterocolitis
35.
Ranking probability (rankogram) of each treatment modality for necrotizing enterocolitis
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Sensitivity analysis
We conducted a sensitivity analysis of studies that specifically reported on infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). 4 studies (n = 1344) that compared indomethacin versus placebo and 3 studies (n = 323) that compared ibuprofen versus placebo reported on necrotizing enterocolitis in infants in this specific gestational age and/or birth weight. Bayesian random effects network meta‐analysis showed no statistically significant difference between indomethacin versus placebo (Network RR 0.95, 95% CrIs 0.32, 2.4) as well as ibuprofen versus placebo (Network RR 1.0, 95% CrIs 0.30, 3.0) for the outcome of necrotizing enterocolitis. There were no differences in the median ranks between any of the interventions [Indomethacin (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.55), ibuprofen (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.48) and placebo (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.47) in this specific gestational age and/or birth weight group.
Gastrointestinal perforation
Four studies (n = 1398) reported on this outcome [Indomethacin versus placebo (2 studies, 1221 infants); ibuprofen versus placebo (2 studies, 177 infants). The network diagram is presented in Figure 36.
36.
Network plot for gastrointestinal perforation
Initial pairwise meta‐analysis using Bayesian random‐effects model showed no statistically significant differences in gastrointestinal perforation with indomethacin compared to placebo (2 studies, 1221 infants; RR 1.1, 95% CrI 0.66 to 1.7) (Figure 37) or ibuprofen versus placebo (2 studies, 177 infants; RR 2.7, 95% CrI 0.40 to 18.00) (Figure 38).
37.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for gastrointestinal perforation
A RR<1 favors the intervention. CrI, Credible intervals
38.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for gastrointestinal perforation
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed no statistically significant difference in gastrointestinal perforation with indomethacin (Network RR 0.92, 95% CrIs 0.11, 3.9; moderate‐certainty) or ibuprofen (Network RR 2.6, 95% CrIs 0.42, 20; very low‐certainty) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 39, Table 7. We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Indomethacin (median rank 1, 95% CrI 1 to 3, mean SUCRA 0.70) ranked as the best treatment for reduction in gastrointestinal perforation (Figure 40).
39.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for gastrointestinal perforation
A RR<1 favors the intervention. CrI, Credible intervals
6. Network effect estimates and ranking statistics for gastrointestinal perforation.
|
Ibuprofen Mean SUCRA, 0.15; median rank, 3 (95% CrI, 1‐3) |
||
| 2.98 ( 0.30, 55.5) |
Indomethacin Mean SUCRA, 0.70; median rank, 1 (95% CrI, 1‐3) |
|
| 2.6 (0.42, 20) | 0.92 (0.11, 3.9) |
Placebo Mean SUCRA, 0.65; median rank, 2 (95% CrI, 1‐3) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
40.
Ranking probability (rankogram) of each treatment modality for gastrointestinal perforation
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Sensitivity Analysis
All four studies mentioned above were conducted in infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). Therefore, no separate sensitivity analysis was conducted for this outcome.
Chronic lung disease (CLD) (defined as use of oxygen or respiratory support at 36 weeks’ postmenstrual age)
Eighteen studies (n = 3058) reported on this outcome [Indomethacin versus placebo (10 studies, 2106 infants); ibuprofen versus placebo (7 studies, 904 infants) and acetaminophen versus placebo (1 study, 48 infants)]. The acetaminophen node had zero events for CLD and therefore was removed from the network meta‐analysis as no continuity correction was applied. The network diagram is presented in Figure 41.
41.
Network plot for chronic lung disease
Initial pairwise meta‐analysis using Bayesian random‐effects model showed no statistically significant differences in CLD with indomethacin compared to placebo (10 studies, 2106 infants; RR 1.1, 95% CrI 0.91 to 1.3) (Figure 42) or ibuprofen versus placebo (7 studies, 904 infants; RR 1.00, 95% CrI 0.74 to 1.4) (Figure 43).
42.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for chronic lung disease
A RR<1 favors the intervention. CrI, Credible intervals
43.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for chronic lung disease
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed no statistically significant difference in CLD with indomethacin (Network RR 1.09, 95% CrIs 0.93, 1.29; low‐certainty) or ibuprofen (Network RR 1.05, 95% CrIs 0.83, 1.32; low‐certainty) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 44, Table 8. Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 45). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Placebo (median rank 1, 95% CrI 1 to 3, mean SUCRA 0.77) ranked as the best option for reduction in CLD followed by ibuprofen (median rank 2, 95% CrI 1 to 3, mean SUCRA 0.47) (Figure 46).
44.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for chronic lung disease
A RR<1 favors the intervention. CrI, Credible intervals
7. Network effect estimates and ranking statistics for chronic lung disease.
|
Ibuprofen Mean SUCRA, 0.47; median rank, 2 (95% CrI, 1‐3) |
||
| 0.96 ( 0.72, 1.26) |
Indomethacin Mean SUCRA, 0.25; median rank, 3 (95% CrI, 1‐3) |
|
| 1.05 ( 0.83, 1.32) | 1.10 ( 0.93, 1.29) |
Placebo Mean SUCRA, 0.77; median rank, 1 (95% CrI, 1‐3) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
45.
Comparison‐adjusted funnel plot for chronic lung disease
46.
Ranking probability (rankogram) of each treatment modality for chronic lung disease
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Sensitivity Analysis
We conducted a sensitivity analysis of studies that specifically reported on infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). 4 studies (n = 1179) that compared indomethacin versus placebo and 3 studies (n = 322) that compared ibuprofen versus placebo reported on chronic lung disease in infants in this specific gestational age and/or birth weight. Bayesian random effects network meta‐analysis showed no statistically significant difference between indomethacin versus placebo (Network RR 1.2, 95% CrIs 0.88, 1.9) as well as ibuprofen versus placebo (Network RR 0.99, 95% CrIs 0.60, 1.7) for the outcome of chronic lung disease. Ibuprofen (median rank 1, 95% CrI 1 to 3; mean SUCRA, 0.65) ranked as the best treatment for reduction in chronic lung disease followed by placebo (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.70) and indomethacin (median rank 3, 95% CrI 1 to 3; mean SUCRA, 0.15) in this specific gestational age and/or birth weight group.
Oliguria
Twelve studies (n = 2864) reported on this outcome [Indomethacin versus placebo (8 studies, 2115 infants); ibuprofen versus placebo (3 studies, 701 infants) and acetaminophen versus placebo (1 study, 48 infants)]. The network diagram is presented in Figure 47.
47.
Network plot for oliguria
Initial pairwise meta‐analysis using Bayesian random‐effects model showed a statistically significant increase in oliguria with indomethacin versus placebo (8 studies, 2115 infants; RR 1.7, 95% CrI 1.2 to 2.4) (Figure 48). No statistically significant difference in oliguria was noted with ibuprofen versus placebo (3 studies, 701 infants; RR 1.3, 95% CrI 0.83 to 2.1) (Figure 49), or with acetaminophen versus placebo (1 study, 48 infants; RR 0.78, 95% CrI 0.28 to 2.16).
48.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for oliguria
A RR<1 favors the intervention. CrI, Credible intervals
49.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for oliguria
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random effects network meta‐analysis showed a statistically significant increase in oliguria with indomethacin (Network RR 1.7, 95% CrIs 1.2, 2.3) (Figure 50). No statistically significant differences in oliguria were noted with ibuprofen (Network RR 1.32, 95% CrIs 0.85, 2.02) or acetaminophen (Network RR 0.68, 95% CrIs 0.20, 1.97) compared to placebo (Figure 50). The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Table 9. Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 51). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Acetaminophen (median rank 1, 95% CrI 1 to 4) ranked as the best treatment option for the outcome of oliguria followed by placebo (median rank 2, 95% CrI 1 to 3), ibuprofen (median rank 3, 95% CrI 1 to 4) and lastly indomethacin (median rank 4, 95% CrI 3 to 4) (Figure 52). Based on the mean SUCRA values, acetaminophen had the highest SUCRA (0.86).
50.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for oliguria
A RR<1 favors the intervention. CrI, Credible intervals
8. Network effect estimates and ranking statistics for oliguria.
|
Acetaminophen Mean SUCRA, 0.86; median rank, 1 (95% CrI, 1‐4) |
|||
| 0.52 ( 0.14, 1.62) |
Ibuprofen Mean SUCRA, 0.35; median rank, 3 (95% CrI, 1‐4) |
||
| 0.40 ( 0.12, 1.23) | 0.78 ( 0.46, 1.34) |
Indomethacin Mean SUCRA, 0.08; median rank, 4 (95% CrI, 3‐4) |
|
| 0.68 ( 0.20, 1.97) | 1.32 ( 0.85, 2.02) | 1.69 ( 1.20, 2.29) |
Placebo Mean SUCRA, 0.71; median rank, 2 (95% CrI, 1‐3) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
51.
Comparison‐adjusted funnel plot for oliguria
52.
Ranking probability (rankogram) of each treatment modality for oliguria
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Intraventricular haemorrhage (IVH) of any grade
Twenty‐two studies (n = 3543) reported on this outcome [Indomethacin versus placebo (16 studies, 2674 infants); ibuprofen versus placebo (5 studies, 821 infants) and acetaminophen versus placebo (1 study, 48 infants)]. The network diagram is presented in Figure 53.
53.
Network plot for intraventricular hemorrhage (any grade)
Initial pairwise meta‐analysis using Bayesian random‐effects model showed a statistically significant reduction in IVH (any grade) with indomethacin versus placebo (16 studies, 2674 infants; RR 0.75, 95% CrI 0.61 to 0.92) (Figure 54). No statistically significant difference in IVH (any grade) was noted with ibuprofen versus placebo (5 studies, 821 infants; RR 0.93, 95% CrI 0.63 to 1.4) (Figure 55), or with acetaminophen versus placebo (1 study, 48 infants; RR 0.65, 95% CrI 0.28 to 1.54).
54.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for intraventricular hemorrhage (any grade)
A RR<1 favors the intervention. CrI, Credible intervals
55.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for intraventricular hemorrhage (any grade)
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed a statistically significant reduction in IVH (any grade) with indomethacin (Network RR 0.77, 95% CrIs 0.62, 0.90) (Figure 56). No statistically significant differences in IVH (any grade) were noted with ibuprofen (Network RR 0.94, 95% CrIs 0.66, 1.31) or acetaminophen (Network RR 0.60, 95% CrIs 0.20, 1.59) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 56, Table 10. Comparison‐adjusted funnel plots were not suggestive of any small‐study effects (Figure 57). We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Acetaminophen (median rank 1, 95% CrI 1 to 4) ranked as the best treatment option for reduction of IVH (any grade) followed by indomethacin (median rank 2, 95% CrI 1 to 3), ibuprofen (median rank 3, 95% CrI 1 to 4) and placebo (median rank 4, 95% CrI 2 to 4) (Figure 58). Based on the mean SUCRA values, acetaminophen had the highest SUCRA (0.78).
56.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for intraventricular hemorrhage (any grade)
A RR<1 favors the intervention. CrI, Credible intervals
9. Network effect estimates and ranking statistics for intraventricular hemorrhage (any grade).
|
Acetaminophen Mean SUCRA, 0.78; median rank, 1 (95% CrI, 1‐4) |
|||
| 0.64 ( 0.21, 1.81) |
Ibuprofen Mean SUCRA, 0.33; median rank, 3 (95% CrI, 1‐4) |
||
| 0.79 ( 0.26, 2.14) | 1.22 ( 0.84, 1.83) |
Indomethacin Mean SUCRA, 0.73; median rank, 2 (95% CrI, 1‐3) |
|
| 0.60 ( 0.20, 1.59) | 0.94 ( 0.66, 1.31) | 0.77 ( 0.62, 0.90) |
Placebo Mean SUCRA, 0.16; median rank, 4 (95% CrI, 2‐4) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
57.
Comparison‐adjusted funnel plot for intraventricular hemorrhage (any grade)
58.
Ranking probability (rankogram) of each treatment modality for intraventricular hemorrhage (any grade)
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Periventricular leukomalacia (PVL) of any grade)
Eight studies (n = 2216) reported on this outcome [Indomethacin versus placebo (4 studies, 1469 infants); ibuprofen versus placebo (4 studies, 747 infants). The network diagram is presented in Figure 59.
59.
Network plot for periventricular leukomalacia
Initial pairwise meta‐analysis using Bayesian random‐effects model showed no statistically significant differences in PVL with indomethacin compared to placebo (4 studies, 1469 infants; RR 0.69, 95% CrI 0.29 to 1.6) (Figure 60) or with ibuprofen versus placebo (4 studies, 747 infants; RR 0.94, 95% CrI 0.46 to 1.9) (Figure 61).
60.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for periventricular leukomalacia
A RR<1 favors the intervention. CrI, Credible intervals
61.
Forest plot of pairwise meta‐analysis between ibuprofen and placebo (conducted using Bayesian random‐effects model) for periventricular leukomalacia
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random effects network meta‐analysis showed no statistically significant difference in PVL with indomethacin (Network RR 0.74, 95% CrIs 0.30, 1.35) or ibuprofen (Network RR 0.94, 95% CrIs 0.40, 2.02) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 62, Table 11. We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Indomethacin (median rank 1, 95% CrI 1 to 3) ranked as the best treatment option for PVL followed by ibuprofen (median rank 2, 95% CrI 1 to 3) and placebo (median rank 2, 95% CrI 1 to 3)(Figure 63). Based on the mean SUCRA values, indomethacin had the highest SUCRA (0.80).
62.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for periventricular leukomalacia
A RR<1 favors the intervention. CrI, Credible intervals
10. Network effect estimates and ranking statistics for periventricular leukomalacia (any grade).
|
Ibuprofen Mean SUCRA, 0.43; median rank, 2 (95% CrI, 1‐3) |
||
| 1.30 ( 0.46, 4.16) |
Indomethacin Mean SUCRA, 0.80; median rank, 1 (95% CrI, 1‐3) |
|
| 0.94 ( 0.40, 2.02) | 0.74 ( 0.30, 1.35) |
Placebo Mean SUCRA, 0.28; median rank, 2 (95% CrI, 1‐3) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
63.
Ranking probability (rankogram) of each treatment modality for periventricular leukomalacia
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Neurodevelopmental outcome (at 18 to 24 months of age)
Due to absence of data on multiple COX‐I drugs network meta‐analysis was not possible for this outcome.
Cerebral palsy (CP)
Five studies (n =1402) reported on this outcome [Indomethacin versus placebo (4 studies, 1367 infants); acetaminophen versus placebo (1 study, 35 infants). The network diagram is presented in Figure 64.
64.
Network plot for cerebral palsy
Initial pairwise meta‐analysis using Bayesian random‐effects model showed no statistically significant differences in CP with indomethacin compared to placebo (4 studies, 1367 infants; RR 0.97, 95% CrI 0.46 to 2.0) (Figure 65), or with acetaminophen versus placebo (1 study, 35 infants; RR 0.84, 95% CrI 0.05 to 13.75).
65.
Forest plot of pairwise meta‐analysis between indomethacin and placebo (conducted using Bayesian random‐effects model) for cerebral palsy
A RR<1 favors the intervention. CrI, Credible intervals
Bayesian random‐effects network meta‐analysis showed no statistically significant difference in CP with indomethacin (Network RR 0.97, 95% CrIs 0.44, 2.11; low‐certainty) or acetaminophen (Network RR 0.36, 95% CrIs 0.01, 6.31; very low‐certainty) compared to placebo. The relative treatment effects for all possible comparisons obtained from the network meta‐analysis are shown in Figure 66; Table 12. We were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Acetaminophen (median rank 1, 95% CrI 1 to 3) ranked as the best treatment option for CP followed by indomethacin (median rank 2, 95% CrI 1 to 3) and placebo (median rank 2, 95% CrI 1 to 3)(Figure 67). Based on the mean SUCRA values, acetaminophen had the highest SUCRA (0.76).
66.
Forest plot of the relative network effect estimates with placebo as the comparator (conducted using Bayesian random‐effects model) for cerebral palsy
A RR<1 favors the intervention. CrI, Credible intervals
11. Network effect estimates and ranking statistics for cerebral palsy.
|
Acetaminophen Mean SUCRA, 0.76; median rank, 1 (95% CrI, 1‐3) |
||
| 0.38 ( 0.01, 6.97) |
Indomethacin Mean SUCRA, 0.39; median rank, 2 (95% CrI, 1‐3) |
|
| 0.36 ( 0.01, 6.31) | 0.97 ( 0.44, 2.11) |
Placebo Mean SUCRA, 0.35; median rank, 2 (95% CrI, 1‐3) |
The unlabeled data in the boxes are risk ratios (RRs) and 95% credible intervals (CrIs). A RR >1 suggests that the upper left treatment is associated with a higher risk of having the outcome of interest vs the corresponding lower right treatment and the opposite is true for an RR <1. SUCRA, surface under the cumulative ranking curve
67.
Ranking probability (rankogram) of each treatment modality for cerebral palsy
Each rank is represented by a color. The height of each colored bar corresponds to the probability of an intervention being ranked in that specific ranking position
Major neurodevelopmental disability
Due to absence of data on multiple COX‐I drugs network meta‐analysis was not possible for this outcome.
Network meta‐regression
The included studies were conducted between 1985 and 2018. Therefore, as planned a priori, we conducted a network meta‐regression, assuming a common fixed coefficient across comparisons to explore the effect of year of publication on the following clinical outcomes:
Severe Intraventricular haemorrhage (IVH)
Bayesian random effects network meta‐regression showed that indomethacin significantly reduced severe IVH compared to placebo (Network RR 0.59, 95% CrIs 0.39, 0.80). There were no statistically significant differences observed with either Ibuprofen (Network RR 0.64, 95% CrIs 0.34, 1.1) or acetaminophen (Network RR 0.48, 95% CrIs 0.02, 6.6) compared to placebo. Acetaminophen (median rank 1, 95% CrI 1 to 4; mean SUCRA, 0.60) had the best median rank for reduction of severe IVH followed by indomethacin (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.68), ibuprofen (median rank 2, 95% CrI 1 to 4; mean SUCRA, 0.59) and placebo (median rank 4, 95% CrI 3 to 4; mean SUCRA, 0.12).
Mortality
Bayesian random effects network meta‐regression showed no statistically significant differences in mortality with indomethacin (Network RR 0.85, 95% CrIs 0.61, 1.1), ibuprofen (Network RR 0.81, 95% CrIs 0.54, 1.2) or acetaminophen (Network RR 0.45, 95% CrIs 0.13, 1.5) compared to placebo. Acetaminophen (median rank 1, 95% CrI 1 to 4; mean SUCRA, 0.85) had the best median rank for reduction of mortality followed by ibuprofen (median rank 2, 95% CrI 1 to 4; mean SUCRA, 0.53), indomethacin (median rank 3, 95% CrI 1 to 4; mean SUCRA, 0.51) and placebo (median rank 4, 95% CrI 2 to 4; mean SUCRA, 0.12).
Chronic lung disease (CLD)
Bayesian random‐effects network meta‐regression showed no statistically significant differences in CLD with indomethacin (Network RR 1.1, 95% CrIs 0.94, 1.5) or ibuprofen (Network RR 0.96, 95% CrIs 0.65, 1.3) compared to placebo. Ibuprofen (median rank 1, 95% CrI 1 to 3; mean SUCRA, 0.70) had the best median rank for reduction of CLD followed by placebo (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.66) and indomethacin (median rank 3, 95% CrI 1 to 3; mean SUCRA, 0.14).
Necrotizing enterocolitis (NEC)
Bayesian random effects network meta‐regression showed no statistically significant differences in NEC with indomethacin (Network RR 0.73, 95% CrIs 0.32, 1.2) or ibuprofen (Network RR 0.74, 95% CrIs 0.26, 1.7) compared to placebo. Indomethacin (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.68) and ibuprofen (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.63) had the best median ranks for reduction of NEC followed by placebo (median rank 3, 95% CrI 2 to 3; mean SUCRA, 0.19).
Gastrointestinal perforation
Bayesian random effects network meta‐regression showed no statistically significant differences in gastrointestinal perforation with indomethacin (Network RR 0.61, 95% CrIs 0.04, 4.1) or ibuprofen (Network RR 2.7, 95% CrIs 0.43, 22.0) compared to placebo. Indomethacin (median rank 1, 95% CrI 1 to 3; mean SUCRA, 0.79) had the best median rank for reduction of gastrointestinal perforation followed by placebo (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.58) and ibuprofen (median rank 3, 95% CrI 1 to 3; mean SUCRA, 0.13).
Planned sensitivity analysis
We did not perform the planned sensitivity analysis including only low risk of bias studies as majority of information in all the three networks (indomethacin versus placebo, ibuprofen versus placebo and acetaminophen versus placebo) was derived from studies at low risk of bias with minimal statistical heterogeneity demonstrated in the direct comparisons.
Discussion
Summary of main results
Twenty‐eight randomized controlled trials (RCTs) completed to date have reported on 3999 infants. Nineteen studies that enrolled 2877 infants compared prophylactic indomethacin versus placebo/no treatment, seven studies that enrolled 914 infants compared prophylactic ibuprofen versus placebo/no treatment and two studies that enrolled 208 infants compared prophylactic acetaminophen versus placebo/no treatment. No head‐to‐head RCTs that directly compared two or more of the three active interventions were identified for inclusion in our review.
Based on the decision thresholds defined by the authoring team, Bayesian random‐effects network meta‐analysis (NMA) of eligible RCTs showed that prophylactic indomethacin probably results in a small reduction in severe intraventricular haemorrhage (IVH), a moderate reduction in mortality and need for surgical patent ductus arteriosus (PDA) closure (moderate certainty). Prophylactic indomethacin may result in a small increase in chronic lung disease (CLD) (low certainty) and results in trivial differences in necrotizing enterocolitis (NEC) (high certainty), gastrointestinal perforation (moderate certainty) and cerebral palsy (low certainty) compared to placebo or no treatment.
Prophylactic ibuprofen probably results in a small reduction in severe IVH and a moderate reduction in need for surgical PDA closure (moderate certainty). Prophylactic ibuprofen may also result in a moderate reduction in mortality (low certainty), and trivial differences in CLD (low certainty) and NEC (high certainty) compared to placebo or no treatment.
The evidence is very uncertain about the effect of acetaminophen on any of the clinically relevant outcomes. Indirect comparisons, where possible, between the three cyclooxygenase inhibitors (COX‐I) drugs revealed no statistically significant differences for any of the clinical outcomes.
Overall completeness and applicability of evidence
This is the first systematic review and NMA comparing prophylactic COX‐I drugs in preterm infants. We used Bayesian random‐effects NMA to derive relative treatment effects and relative treatment rankings for the four possible pharmacoprophylactic options (indomethacin, ibuprofen, acetaminophen, and placebo/no treatment) for each clinical outcome, where possible. Although the use of NMA has allowed us to derive more precise effect estimates for each of the COX‐I drugs versus placebo and to generate effect estimates against each other through indirect comparisons, we recommend cautious interpretation of the relative treatment rankings, especially for acetaminophen.
This is primarily due to the fact that majority of the evidence in the network was contributed by randomized controlled trials comparing indomethacin versus placebo (19 studies, 2877 infants) and ibuprofen versus placebo (7 studies, 914 infants). Only 208 participants out of 3999 in the entire network were contributed by studies that used prophylactic acetaminophen (2 studies). This has resulted in imprecise effect estimates for acetaminophen. Although this imprecision is adequately accounted for in the GRADE certainty of evidence, resulting in very low certainty for all the acetaminophen estimates, the median ranks and surface under the cumulative ranking curve (SUCRA) values in such sparse networks could be misleading. For example, for the outcome of mortality, acetaminophen ranks as the best intervention (median rank 1) ahead of indomethacin and ibuprofen, with the best mean SUCRA value (0.87). This is primarily because the network risk ratio (RR) point estimate for acetaminophen (0.49) is substantially better than either indomethacin (0.85) or ibuprofen (0.83). However, the median rank and mean SUCRA value fail to account for the imprecision around this point estimate (acetaminophen network RR for mortality: 0.49, 95% credible intervals (CrIs) 0.16 to 1.4), which is demonstrated by the 95% CrIs around the median rank (1‐4, in the case of acetaminophen for mortality). Therefore, simply stating that acetaminophen is the best intervention for the critical outcome of mortality would be an oversimplification of the interpretation of NMA results. Hence, readers should consider the imprecision (95% CrIs) around the network effect estimates and median ranks while determining the relative benefit or harm of an intervention with respect to a particular outcome.
Subgroup considerations
There is considerable debate on the use of prophylactic COX‐I drugs in preterm infants. Based on existing evidence, the American Academy of Pediatrics (Hamrick 2020) and the Canadian Pediatric Society (Ryan 2019) recently suggested considering the use of prophylactic indomethacin in extremely low gestational age neonates (ELGANs, born less than 28 weeks of gestational age), or extremely low birth weight (ELBW, birth weight less than 1000 g) infants, especially if they are at a high risk of severe IVH (such as gestational age at birth <2 6 weeks, lack of antenatal corticosteroids, and male sex). We conducted a sensitivity analysis to specifically explore the effect of COX‐I drugs in ELGAN and/or ELBW infants. The notable differences with the primary analysis results that may affect clinical decision‐making on prophylactic indomethacin use were the following.
a) Severe IVH: prophylactic indomethacin no longer had a statistically significant benefit for reduction of severe IVH in this group (Network RR 0.81, 95% CrIs 0.37, 2.0). Prophylactic ibuprofen (Network RR 0.46, 95% CrIs 0.14, 1.2) ranked higher (median rank 1, 95% CrI 1 to 3; mean SUCRA, 0.91) than prophylactic indomethacin (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.43) in this gestational age and/or birth weight group. This result might be an important practice consideration for centres that routinely use prophylactic indomethacin for prevention of IVH in extremely preterm or ELBW infants.
b) Mortality: similar to the results of severe IVH above, prophylactic indomethacin no longer demonstrated a statistically significant benefit for reduction in mortality in this gestational age/birthweight (GA/BW) group (Network RR 1.2, 95% CrIs 0.74, 1.9). Both prophylactic ibuprofen (median rank 1, 95% CrI 1 to 3; mean SUCRA, 0.87) as well as placebo (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.48) ranked higher than prophylactic indomethacin (median rank 3, 95% CrI 1 to 3; mean SUCRA, 0.15) in this specific gestational age and/or birth weight group.
c) Surgical PDA closure: prophylactic indomethacin no longer demonstrated a statistically significant reduction in need for surgical PDA closure in this GA/BW group (Network RR 0.56, 95% CrIs 0.13, 3.0). Prophylactic ibuprofen (Network RR 0.07, 95% CrIs 0.001, 0.73) still demonstrated a statistically significant reduction in need for PDA ligation and therefore maintained a higher rank (median rank 1, 95% CrI 1 to 2; mean SUCRA, 0.97) than prophylactic indomethacin (median rank 2, 95% CrI 1 to 3; mean SUCRA, 0.45) in this specific gestational age and/or birth weight group.
Moreover, both the primary and the sensitivity analysis demonstrated that indomethacin ranked as the least preferable option for reduction of CLD. Given that prophylactic indomethacin is unlikely to significantly reduce severe IVH, mortality or surgical PDA ligation and, in addition may lead to a small increase in risk of CLD, caution should be exercised while considering routine use of prophylactic indomethacin in ELGAN and/or ELBW infants. Current evidence, thus, fails to demonstrate benefit of any of the COX‐I drugs in improving critical outcomes such as severe IVH or mortality in ELGAN and/or ELBW infants.
Quality of the evidence
The certainty of evidence for the primary outcome of severe IVH was moderate for the comparisons of indomethacin versus placebo and ibuprofen versus placebo while it was very low for acetaminophen versus placebo. The certainty of evidence for the primary outcome of mortality was moderate for the comparison of indomethacin versus placebo, low for ibuprofen versus placebo and very low for acetaminophen versus placebo. We used the ‘GRADE guidelines on informative statements to communicate the findings of systematic reviews of interventions’ by Santesso 2020 to formulate statements on the size of the effect estimate and certainty of evidence in our result summaries.
Readers should consider the following while interpreting the certainty of evidence as determined in this review.
a) Imprecision: prior to assessing the certainty of evidence, the authoring team adopted a partially contextualized approach for addressing imprecision in the NMA estimates following the GRADE guidance by Brignardello‐Petersen 2021. We defined thresholds for benefit or harm for each outcome (listed in the protocol for certainty assessment) and assessed the imprecision in the context of these thresholds. For the outcome of mortality, ‘small’ benefit/harm was defined as < 20 fewer or more per 1000, respectively; ‘moderate’ benefit/harm was defined as 20 to 50 fewer or more per 1000, respectively; ‘large’ benefit/harm was defined as > 50 fewer or more per 1000, respectively. For all other outcomes listed in the summary of findings table, any effect < 20 fewer or more per 1000 was defined as a trivial benefit or harm. No direction of effect was specified for trivial effects. A ‘small’ benefit/harm was defined as 20 to 50 fewer or more per 1000, respectively, ‘moderate’ benefit/harm was defined as 50‐100 fewer or more per 1000 respectively and ‘large’ benefit/harm was defined as >100 fewer or more per 1000 respectively. A moderate or large effect was considered as an ‘appreciable’ effect. If the 95% CrIs included an appreciable effect at one end of the 95% CrI (i.e. small benefit‐appreciable harm or small harm‐appreciable benefit), the certainty was rated down by one‐level. If the 95% CrIs included both appreciable benefit and harm, the certainty was rated down by 2 levels. Further, in sparse networks (such as with acetaminophen versus placebo) where the 95% CrIs included implausible benefit/harm, we chose to rate the certainty of evidence down by 3 levels as per the recent GRADE guidance by Brignardello‐Petersen 2021. Decision‐makers and guideline panels may choose to use different decision thresholds and appropriately update the certainty of evidence prior to formulating guideline recommendations.
b) Inconsistency: the networks for none of the outcomes in our review had closed loops as there were no head‐to‐head RCTs between the active interventions; all RCTs had compared an active intervention against placebo/no treatment. Therefore, in the NMA, we were unable to obtain both direct and indirect estimates for any set of comparisons; we either had only direct or only indirect estimates. As a result, we were unable to run any inconsistency models and hence we were unable to judge the NMA inconsistency domain for GRADE. In our protocol we had specified that “when assessment of statistical inconsistency is not possible due to absence of head‐to‐head comparisons between interventions, we will not rate down the certainty of evidence any further due to presumed inconsistency, as the NMA would have been conducted under the strict assumption of transitivity thereby ensuring clinical and methodological homogeneity between the indirect comparisons”. Therefore, the certainty of evidence for none of the comparisons were rated down for inconsistency.
Potential biases in the review process
We are not aware of any biases in the review process. Review authors were not involved with any of the included trials. All included studies strictly met our pre‐defined criteria for transitivity defined by the inclusion of only preterm or low birth weight infants, within the first 72 hours of birth and without a prior clinical or echocardiographic diagnosis of a PDA. However, we were unable to run any inconsistency models as there were no head‐to‐head trials between any of the three COX‐I drugs. Therefore, though the transitivity assumption was met, we could not statistically assess consistency of our NMA models.
Agreements and disagreements with other studies or reviews
Three previous Cochrane Reviews have separately compared placebo/no treatment against prophylactic indomethacin, ibuprofen, or acetaminophen, respectively (Fowlie 2010; Ohlsson 2020b; Ohlsson 2020c). All three previous reviews used a fixed‐effect model for their statistical analysis, whereas we used a Bayesian random‐effects model for both our direct and indirect comparisons.
The review by Fowlie 2010 on use of prophylactic intravenous indomethacin was last updated in 2010 and did not include any assessment of certainty of evidence. The Fowlie 2010 review demonstrated that prophylactic indomethacin resulted in a statistically significant reduction in severe IVH and has subsequently formed the basis of its routine prophylactic use in many neonatal centres. In our review, we found four additional studies (Jannatdoust 2014; Kumar Nair 2004; Maruyama 2012; Vogtmann 1988) comparing prophylactic indomethacin versus placebo that met our inclusion criteria and were added to the indomethacin versus placebo arm. Our overall network effect estimates were similar to those from the Fowlie review, and we also demonstrated that prophylactic indomethacin overall results in a statistically significant reduction in severe IVH. We further added a sensitivity analysis for ELGAN and/or ELBW infants which showed that in this particular subgroup prophylactic indomethacin may not reduce the incidence of severe IVH. This finding may have important practice implications.
The updated review by Ohlsson 2020c on the use of prophylactic ibuprofen included nine trials ( n = 1070) while our review included seven trials (n = 914). Two studies included in the Ohlsson 2020c review were not included in our review, as they did not meet our inclusion criteria. The study by Sangtawesin 2008 included only infants who were diagnosed with a PDA within the first 24 hours after birth which did not meet our definition of prophylactic therapy. The study by Kalani 2016 was placed in Characteristics of studies awaiting classification as their methods section suggested that it was a retrospective study, and we were unable to establish contact with the primary author to clarify this discrepancy. However, the effect estimates and certainty of evidence for clinically relevant outcomes in the Ohlsson 2020c review were similar to our review.
The updated review by Ohlsson 2020b on use of prophylactic acetaminophen included two trials (n = 80), while our review included two trials (n = 208). We did not include the study by Akbari Asbagh 2015 as we were unable to contact the corresponding author to obtain clarifying information on outcome data. Hence, this study has been placed in Characteristics of studies awaiting classification. Due to overall paucity of data, neither the Ohlsson 2020b review nor our review could precisely establish or refute any clinically meaningful benefit/harm with use of prophylactic acetaminophen.
Authors' conclusions
Implications for practice.
Prophylactic indomethacin probably results in a small reduction in severe intraventricular haemorrhage (IVH) and a moderate reduction in mortality and need for surgical patent ductus arteriosus (PDA) closure (moderate certainty), may result in a small increase in chronic lung disease (CLD) (low certainty) and results in trivial differences in necrotizing enterocolitis (NEC) (high certainty), gastrointestinal perforation (moderate certainty) and cerebral palsy (CP) (low certainty) compared to placebo. In the subgroup of extremely preterm and/or extremely low birth weight infants, prophylactic indomethacin is unlikely to reduce severe IVH, mortality, or need for PDA ligation.
Prophylactic ibuprofen probably results in a small reduction in severe IVH and a moderate reduction in need for surgical PDA closure (moderate certainty), may result in a moderate reduction in mortality (low certainty) and trivial differences in CLD (low certainty) and NEC (high certainty) compared to placebo. In the subgroup of extremely preterm and/or extremely low birth weight infants, prophylactic ibuprofen may reduce need for PDA ligation, but is unlikely to reduce severe IVH or mortality.
The evidence is very uncertain about the effect of acetaminophen on any of the clinically relevant outcomes.
Implications for research.
Given that extremely preterm infants born < 26 weeks' of gestation are at the highest risk of mortality and major morbidity such as severe IVH, CLD, NEC and neurodevelopmental impairment, future COX‐I pharmacoprophylaxis trials should be designed to explore the effectiveness and safety of the COX‐I drugs specifically in this high‐risk population. Out of the three COX‐I medications, acetaminophen clearly lacks good quality evidence for its use as pharmacoprophylaxis. Therefore, additional large trials specifically on acetaminophen pharmacoprophylaxis in extremely low gestational age neonates are warranted. There are currently two ongoing randomized controlled trials on prophylactic use of acetaminophen in extremely preterm infants born less than 28 weeks of gestational age (NCT03641209; NCT04459117). In addition, large, well‐designed, prospective observational studies might provide useful data for potential harms of these COX‐I medications in extremely preterm infants. Given the low rate of adverse clinical outcomes, lack of clear benefit and potential for harm with routine use, there is no clinical equipoise for use of prophylactic COX‐I medications in older preterm infants. Therefore, we do not recommend any further research on COX‐I prophylaxis in older preterm infants, especially those born after 28 weeks' of gestation.
History
Protocol first published: Issue 1, 2021
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman (Managing Editor), Jane Cracknell (Assistant Managing Editor), Roger Soll (Co‐coordinating editor), and Bill McGuire (Co‐coordinating Editor), who provided editorial and administrative support.
We would also like to thank Robin Parker (WK Kellogg Health Sciences Library, Dalhousie University), as the creator of these searches, and Leah Boulos (Evidence Synthesis Co‐ordinator, Maritime SPOR SUPPORT Unit), for her peer review of the MEDLINE search strategy.
Menelaos Konstantinidis, Kerry Dwan (Cochrane Methods Support Unit) and Prakeshkumar Shah (Cochrane Neonatal Senior Editor) peer‐reviewed and offered feedback for this protocol.
The methods section of the protocol is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Search strategies
Medline search strategy
Ovid MEDLINE(R) ALL <1946 to 8 December 2021>
| Search history sorted by search number ascending | ||
| # | Searches | Results |
| 1 | exp Infant, Premature/ or Premature Birth/ or Infant, Premature, Diseases/ or (preterm or pre term or prematur* or pre matur* or premie or premies or preemie*).ti,ab,kf. | 243881 |
| 2 | low birth weight.ti,ab,kf. or Infant, Low Birth Weight/ | 39717 |
| 3 | very low birth weight.ti,ab,kf. or Infant, Very Low Birth Weight/ | 12850 |
| 4 | Infant, Extremely Low Birth Weight/ or (elbw or vlbw or lbw).ti,ab,kf. | 10790 |
| 5 | ((("37" or "36" or "35" or "34" or "33" or "32" or "31" or "30" or "29" or "28" or "27" or "26") adj1 (week? or wk?)) and (birth or neonat* or age or gestat* or pregnan*)).ti,ab,kf. | 68360 |
| 6 | 1 or 2 or 3 or 4 or 5 | 300088 |
| 7 | exp Cyclooxygenase Inhibitors/ | 133274 |
| 8 | exp Anti‐Inflammatory Agents, Non‐Steroidal/ | 206693 |
| 9 | Acetaminophen/ | 19358 |
| 10 | (COXI or Indomethacin or indometacin or indocid or Ibuprofen or brufen or motrin or nuprin or rufen or advil or Ibumetin or Acetaminophen or paracetamol or Tylenol or anephen or acetaco or anacin* or datril or panadol or acamol or algotropyl or NSAID?).ti,ab,kf. | 97044 |
| 11 | ((cyclo‐oxygenase or Cyclooxygenase or Prostaglandin Synthase or Prostaglandin Synthesis or Prostaglandin Endoperoxide Synthase) adj2 (inhibitor* or antagonist*)).ti,ab,kf. | 11988 |
| 12 | ((Anti‐Inflammatory or antiinflammatory or aspirin‐like or nonsteroidal or non‐steroidal) adj2 (Analgesic? or agent? or drug? or medicine? or medication?)).ti,ab,kf. | 68079 |
| 13 | ((Anti‐Inflammatory or antiinflammatory or aspirin‐like or nonsteroid* or non‐steroid*) adj2 (Analgesic? or agent? or drug? or medicine? or medication?)).ti,ab,kf. | 68298 |
| 14 | "Mefenamic Acid".ti,ab,kf. | 1391 |
| 15 | ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. | 1299151 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 14 | 285794 |
| 17 | 6 and 15 and 16 | 922 |
Embase search strategy
| No. | Query | Results |
| #15 | #3 AND #13 AND #14 | 3927 |
| #14 | #4 OR #5 OR #6 OR #7 | 963514 |
| #13 | #8 OR #9 OR #10 OR #11 OR #12 | 1157104 |
| #12 | 'mefenamic acid':ti,ab,kw | 1856 |
| #11 | (('anti‐inflammatory' OR antiinflammatory OR 'aspirin‐like' OR nonsteroid* OR 'non‐steroid*') NEAR/2 (analgesic* OR agent* OR drug* OR medicine* OR medication*)):ti,ab,kw | 94572 |
| #10 | ('cyclo‐oxygenase' OR cyclooxygenase OR 'prostaglandin synthase' OR 'prostaglandin synthesis' OR 'prostaglandin endoperoxide synthase') NEAR/2 (inhibitor* OR antagonist*) | 38175 |
| #9 | 'nonsteroid antiinflammatory agent'/exp OR 'prostaglandin synthase inhibitor'/exp OR 'paracetamol'/de OR 'ibuprofen'/de OR 'indometacin'/de | 1113616 |
| #8 | coxi:ti,ab,kw OR indomethacin:ti,ab,kw OR indometacin:ti,ab,kw OR indocid:ti,ab,kw OR ibuprofen:ti,ab,kw OR brufen:ti,ab,kw OR motrin:ti,ab,kw OR nuprin:ti,ab,kw OR rufen:ti,ab,kw OR advil:ti,ab,kw OR ibumetin:ti,ab,kw OR acetaminophen:ti,ab,kw OR paracetamol:ti,ab,kw OR tylenol:ti,ab,kw OR anephen:ti,ab,kw OR acetaco:ti,ab,kw OR anacin*:ti,ab,kw OR datril:ti,ab,kw OR panadol:ti,ab,kw OR acamol:ti,ab,kw OR algotropyl:ti,ab,kw OR nsaid*:ti,ab,kw | 144274 |
| #7 | ((('37' OR '36' OR '35' OR '34' OR '33' OR '32' OR '31' OR '30' OR '29' OR '28' OR '27' OR '26') NEAR/1 (week* OR wk*)):ti,ab,kw) AND (birth:ti,ab,kw OR neonat*:ti,ab,kw OR age:ti,ab,kw OR gestat*:ti,ab,kw OR pregnan*:ti,ab,kw) | 104783 |
| #6 | 'immature and premature labor'/exp | 169587 |
| #5 | preterm:ti,ab,kw OR 'pre term':ti,ab,kw OR prematur*:ti,ab,kw OR 'pre matur*':ti,ab,kw OR premie:ti,ab,kw OR premies:ti,ab,kw OR preemie*:ti,ab,kw OR 'low birth weight':ti,ab,kw OR lbw:ti,ab,kw OR vlbw:ti,ab,kw OR elbw:ti,ab,kw | 327721 |
| #4 | 'prematurity'/exp OR 'very low birth weight'/exp OR 'low birth weight'/exp OR 'extremely low birth weight'/exp OR 'premature labor'/exp OR 'newborn'/exp | 758481 |
| #3 | #1 OR #2 | 2986848 |
| #2 | 'controlled clinical trial'/exp | 864591 |
| #1 | 'crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR random*:de,ab,ti,kw OR factorial*:de,ab,ti,kw OR crossover*:de,ab,ti,kw OR ((cross NEXT/1 over*):de,ab,ti,kw) OR placebo*:de,ab,ti,kw OR ((doubl* NEAR/1 blind*):de,ab,ti,kw) OR ((singl* NEAR/1 blind*):de,ab,ti,kw) OR assign*:de,ab,ti,kw OR allocat*:de,ab,ti,kw OR volunteer*:de,ab,ti,kw | 2856698 |
Cochrane CENTRAL search strategy
Cochrane CENTRAL via Cochrane Library (Wiley Issue 12, December 2021)
ID Search
#1 [mh "Infant, premature"] OR [mh "Premature Birth"] OR [mh "Infant,Premature,Diseases"] OR (preterm or pre term or premature* or pre matur* or premie or premies or preemie*):ti,ab,kw 43617
#2 ("low birth weight" OR Infant):ti,ab,kw OR [mh "Low Birth Weight"] 55060
#3 ("very low birth weight" OR Infant):ti,ab,kw OR [mh "Very low birth weight"] 54005
#4 [mh "infant, extremely low birth weight"] OR ("extremely low birth weight" OR elbw OR vlbw OR lbw):ti,ab,kw 2077
#5 ((("37" OR "36" OR "35" OR "34" OR "33" OR "32" OR "31" OR "30" OR "29" OR "28" OR "27" OR "26") NEAR/1 (week? OR wk?)) AND (birth OR neonat* OR age OR gestat* OR pregnan*)):ti,ab,kw 18607
#6 #1 OR #2 OR #3 OR #4 OR #5 95858
#7 [mh "Cyclooxygenase Inhibitors"] 1581
#8 [mh "Anti‐Inflammatory Agents, Non‐Steroidal"] 7833
#9 [mh ^"Acetaminophen"] 3403
#10 (COXI or Indomethacin or indometacin or indocid or Ibuprofen or brufen or motrin or nuprin or rufen or advil or Ibumetin or Acetaminophen or paracetamol or Tylenol or anephen or acetaco or anacin* or datril or panadol or acamol or algotropyl or NSAID?):ti,ab,kw 23089
#11 (("cyclo‐oxygenase" or Cyclooxygenase or "Prostaglandin Synthase" or "Prostaglandin Synthesis" or "Prostaglandin Endoperoxide Synthase") NEAR/2 (inhibitor* or antagonist*)):ti,ab,kw 2227
#12 ((Anti‐Inflammatory or antiinflammatory or aspirin‐like or nonsteroidal or non‐steroidal) NEAR/2 (Analgesic? or agent? or drug? or medicine? or medication?)):ti,ab,kw 21861
#13 "Mefenamic Acid":ti,ab,kw 462
#14 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 39494
#15 #6 and #14 2414 =>2281 CENTRAL
Custom Date Range: 01102020 – 09122021 = 113
Trial registry and conference abstract search strategies
US National Library of Medicine (clinicaltrials.gov)
Search terms:
condition: premature AND other terms: Prong = 54 [Limit Child]
condition: neonate AND other terms: Prong = 51 [Limit Child]
Condition: premature AND Other terms: cpap = 278 [Limit Child]
Conditon: neonate AND Other terms: cpap = 263 [Limit Child]
Total: 646
Duplicates: 326
Net: 320
Conference websites:35
Appendix 2. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we sought information regarding the method of randomization, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as being at a low, high, or unclear risk of bias. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the ‘Characteristics of included studies’ table.
We evaluated the following issues and entered the findings into the ’Risk of bias’ table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as being at:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as being at:
low, high, or unclear risk of bias for participants; and
low, high, or unclear risk of bias for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as being at:
low risk of bias for outcome assessors;
high risk of bias for outcome assessors; or
unclear risk of bias for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where enough information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorized the methods as being at:
low risk of bias (less than 20% missing data);
high risk of bias (20% missing data or greater); or
unclear risk of bias.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus the outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as being at:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more of the reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so cannot be used; or where the study fails to include results of a key outcome that one would expect to have been reported); or
unclear risk of bias.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we will described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process).
We assessed whether each study was at:
low risk of other sources of bias;
high risk of other sources of bias; or
unclear risk of other sources of bias.
If needed, we planned to undertake sensitivity analyses to explore the impact of the level of bias.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bada 1989.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 71) Prophylactic IV indomethacin 0.2 mg/kg at 6 hours of age; and 0.1 mg/kg at 18 hours and 30 hours of age Control (n = 70) IV placebo (no description available) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Regional Medical Center, Memphis, Tenessee, USA Study period: not specified Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was not stated how randomization was done |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used suggesting personnel were blinded during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome assessed by one investigator blinded to the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants accounted for |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for comparison |
| Other bias | Unclear risk | No specific issues noted. |
Bagheri 2018.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 80) Prophylactic IV acetaminophen, 1st dose 20 mg/kg at 12 hours, then 7.5 mg/kg every 6 hours up to <4 days old Control (n = 80) No placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Kerman, Iran Study period: November 2015 to November 2016 Trial registration: IR.KMU.REC.1395.841 and IRCT2017012718994N2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was not stated how randomization sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors say the nurses giving injections were unaware of case‐control division as paracetamol can be used as analgesic. Following first dose of paracetamol the infants were examined closely for any new symptoms prompting exclusion or further testing. This detailed examination would not have occurred in the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cardiologists evaluating echocardiograms were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The trial was registered with Iranian Registry of Clinical Trials (IRCT2017012718994N2) in 2017 retrospectively following complete recruitment (2015‐2016). There does not seem to be any obvious protocol deviations. |
| Other bias | Low risk | none noted |
Couser 1996.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 43) Prophylactic IV indomethacin sodium trihydrate (Indocin) 0.1mg/kg every 24 hours for 6 doses slow IV infusion over 20 minutes; initiated within 24 hours of birth Control (n = 47) IV placebo (0.9% saline solution given at same times as indomethacin treatment group) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Abbott‐Northwestern Hospital and Children's Health Care,,Minneapolis, USA Study period: 3 June 1994 to 18 Oct 1995 Trial registration: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Individuals administering the treatment were blinded, staff examining and caring for infants were blinded. Hospital pharmacists prepared blinded indomethacin and blinded placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cardiologists blinded to patient assignment and not involved in patient management. Examiners blinded to patient assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 93 enrolled infants accounted for (3 excluded due to ventricular septal defect before analysis). |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable for comparison |
| Other bias | Low risk | Appeared free of other bias. |
Dani 2000.
| Study characteristics | ||
| Methods | Two‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 40) Prophylactic IV ibuprofen lysine (Arfen, Lisapharma, Italy) 10 mg/kg within first 24 hours of life, followed by 5 mg/kg after 24 and 48 hours Control (n = 40) The control group received no prophylactic therapy. The control group received same pharmacological treatment after echocardiographic diagnosis of PDA |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Careggi University Hospital of Florence and Sant'Anna University Hospital of Turin, Italy Study period: February 1995 to January 1996 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method not specified. |
| Allocation concealment (selection bias) | Low risk | sealed envelope technique used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo and no indication of blinding efforts |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear if the assessors for reported outcomes were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all enrolled infants. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable. Unclear if there were any deviations from the protocol. |
| Other bias | Low risk | Appeared free of other bias. |
Dani 2005.
| Study characteristics | ||
| Methods | Multi‐center (7 centres) randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 77) Prophylactic IV ibuprofen lysine; 3 doses (10 mg/kg within 6 hours after birth, followed by 5 mg/kg after 24 and 48 hours). The medications were infused continuously over a 15‐minute period. Control (n = 78) Indistinguishable placebo infused continuously over a 15‐minute period. |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: the primary study location was Careggi University Hospital of Florence, Italy. The study was conducted across 7 tertiary neonatal care units across Italy Study period: February 1995 to January 1996 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation unspecified. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment via sealed‐envelope technique, with envelopes prepared and distributed to participating study sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Indistinguishable placebo was administered to control group to ensure blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were unaware of group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 were excluded after randomization due to incomplete data entry (4 from ibuprofen). No other missing outcome data noted. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable. Unclear if there were any deviations from the protocol. |
| Other bias | Low risk | Appears free of other bias. |
De Carolis 2000.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 23) Prophylactic IV ibuprofen lysine; 3 doses (10 mg/kg within 2 hours after birth, followed by 5 mg/kg after 24 and 48 hours). The medications were infused continuously over a 20‐minute period. Control (n = 23) No placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Catholic University of the Sacred Heart, Rome, Italy Study period: 1 April 1996 to 30 July 1997 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo not used for control group. No mention of other blinding efforts. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Echocardiography outcome assessor was blinded to treatment arm |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. Unclear if any deviations from protocol exist. |
| Other bias | Low risk | Apart from lack of placebo, appears free of other bias. |
Gournay 2004.
| Study characteristics | ||
| Methods | Multi‐center (11 centres) randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 65) Prophylactic IV ibuprofen lysine; loading dose 10 mg/kg followed by 2 maintenance doses of 5 mg/kg at 24‐hour intervals (equivalent volumes for placebo), each infused over 20 minutes Control (n = 66) Blinded IV placebo (2 mL vials with 0.9% saline) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: the primary study location was Nantes, France. The study was conducted across 11 tertiary neonatal care units across France Study period: March 2001 to December 2001 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method not specified. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope allocation kept at hospital pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo (0.9% saline) was used suggesting that personnel were blinded to the allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo (0.9% saline) was used suggesting that outcome assessors were blinded to the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 135 infants were included in the study; 4 infants were not randomized due to errors in study drug allocation (3 mistakenly received open‐label ibuprofen during their prophylactic course, and one 10‐day‐old with diagnosis of PDA was mistakenly given 2 doses of placebo instead of open‐label therapeutic ibuprofen. Per‐protocol analyses were performed on 131 infants. No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The trial was not pre‐registered in any trials registry |
| Other bias | Unclear risk | The study was sponsored by the manufacturers of the intervention drug ibuprofen lysine (Orphan Europe, Paris, France). The sponsors were involved in the study design, data management, data analysis and data interpretation. All final data analyses were double checked by one of the co‐authors (JCR) who had free access to the raw data. |
Hanigan 1988.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 56) Blinded IV indomethacin as reconstituted lyophilized sodium salt; 0.1mg/kg at <12 hours, and 24, 48 and 72 hours IV, over 2 minutes Control (n = 55) Blinded IV placebo (Placebo identical quantity of saline solution) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Illinois, USA Study period: 1 May 1984 to 30 April 1986 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Low risk | Used random‐sized block allocation, and opaque sealed envelopes available only by the pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel involved in care were blinded to participants' study arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States that only biostatistician and pharmacist had access to study arms, implying that outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 infants enrolled were withdrawn from study before statistical analysis, six due to oliguria or thrombocytopenia, one withdrew consent, four due to false‐negative baseline sonograms. No enrolled infants were unaccounted for. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable, unclear if there were any deviations to the original protocol. |
| Other bias | Low risk | None noted |
Harkin 2016.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 23) Blinded IV acetaminophen initiated within 24 hours after birth; loading dose: 20 mg/kg then maintenance dose 7.5 mg/kg every 6 hours for 4 days (given as 15‐minute IV infusions). Control (n = 25) Blinded IV placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Oulu University Hospital, Finland Study period: 18 September 2013 to 2 January 2015 Trial registration: ClinicalTrials.gov: NCT01938261; European Clinical Trials Database: EudraCT 2013‐008142‐33 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computed randomization with 4‐block design was used. |
| Allocation concealment (selection bias) | Low risk | Sealed‐envelop technique used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was placebo‐controlled and all nurses and doctors involved in treatment and study of infants were blinded to study medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All doctors and nurses involved with the study of the infants were blinded to study medication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported for all randomized infants. |
| Selective reporting (reporting bias) | Low risk | Clinical trial was registered with European Clinical Trials Database (2013‐008142‐33) and ClinicalTrials.gov (NCT01938261). Access to ClinicalTrials.gov showed no major deviations from protocol. |
| Other bias | Low risk | Paracetamol preparation changed mid‐study due to hospital protocol. Unlikely to be a source of bias. |
Jannatdoust 2014.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 35) IV indomethacin; initial dose 0.2 mg/kg administered between 2 to 12 hours followed by 2 doses of 0.1 mg/kg each at 24 and 48 hours Control (n = 35) No placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Alzahra Educational‐Medical Center, Tabriz, Iran Study period: June 2010 to December 2012 Trial registration: IRCT201107117010N1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized randomized number generator used |
| Allocation concealment (selection bias) | Low risk | Random allocation determined by Rand List Software |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of placebo use in control group and no mention of blinding efforts. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of placebo use in control group and no mention of blinding efforts. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported for all randomized infants. |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered retrospectively with the Iranian Registry of Clinical Trials (IRCT201107117010N1). |
| Other bias | Unclear risk | Given this was an unblinded study and it was retrospectively registered, difficult to assess if there were other sources of bias. |
Kanmaz 2013.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 23) Oral ibuprofen,10mg/kg within 12 to 24 hours after birth followed by 5 mg/kg at 24 and 48 hours. Control (n = 23) No placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Zekai Tahir Burak Maternity Teaching Hospital, Ankara, Turkey Study period: July 2011 and November 2011 Trial registration: NCT01400737 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method not specified |
| Allocation concealment (selection bias) | Low risk | Patients allocated using sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group received no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cardiologist blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all enrolled infants |
| Selective reporting (reporting bias) | Low risk | All outcomes described in protocol were reported. The trial was registered with ClinicalTrials.gov (NCT01400737). |
| Other bias | Unclear risk | Trial was ended prematurely due to high incidence of adverse effects. |
Krueger 1987.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 15) Indomethacin IV single dose of 0.2mg/kg at 24 hours of age Control (n = 17) No placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Vanderbilt Hospital, Nashville, Tennessee, USA Study period: not reported Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used for control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No placebo was used for control group and there was no indication of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Several infants excluded from analyses following early death, which was clearly described. No other missing outcomes noted. |
| Selective reporting (reporting bias) | Unclear risk | We could not judge if there were any deviations from the original protocol. |
| Other bias | Low risk | No other obvious sources of bias |
Kumar Nair 2004.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 56) Indomethacin IV for a total of 3 doses at 0.1 mg/kg/dose. First dose administered over period of no less than 30 minutes between 6 and 12 hours of age, second and third dose administered at 24‐hour intervals if initial ultrasound detected no IVH. Control (n = 59) No placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Royal Hospital, Oman Study period: March 1998 to March 2001 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple random sampling method used for randomization. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used, mixed up, and stored in locked box. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding or placebo used for control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No evidence of blinding or placebo used for control and no evidence of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data were noted |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for comparison |
| Other bias | Unclear risk | Study terminated prematurely. |
Mahony 1985.
| Study characteristics | ||
| Methods | Single‐centre double‐blind randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 51) Blinded IV Indomethacin, first dose (given at 12 to 18 hours) was 0.2 mg/kg body weight and second dose (given 12 hours later) was 0.1 mg/kg and third dose (given 36 hours after the first) was 0.1 mg/kg. Control (n = 53) Blinded IV placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: James Whitcomb Riley Hospital, Indiana, USA Study period: March 1982 to October 1983 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were randomly allocated by a statistician otherwise uninvolved with the study, however the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by placing identical vials of either indomethacin or placebo into envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Persons evaluating and caring for infants were unaware of study drug assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Allocation of infants was not revealed until after discharge and outcome data collection was complete. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 infants were excluded from the analysis due to death before receiving all 3 doses of study drug. Outcomes were reported for all other randomized infants. |
| Selective reporting (reporting bias) | Unclear risk | We could not judge if there were any deviations from the protocol. |
| Other bias | Unclear risk | Study was stopped early due to lack of power to prove desired results; unclear if this was pre‐specified |
Maruyama 2012.
| Study characteristics | ||
| Methods | Multi‐centre (21 centres) randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 10) IV Indomethacin (0.1 mg/kg/dose) admixed with menatetrenone given as IV for a total of 3 doses (0.0125 mg/mL indomethacin and 0.0625 mg/mL menatetrenone continuous 6 hours IV infusions every 24 hours with first dose within 6 hours of birth) Control (n = 9) IV Placebo (0.0625 mg/mL menatetrenone as a 6‐hour continuous intravenous infusion every 24 hours) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: the primary study location was Gunma Children’s Medical Center, Hokkitsu, Japan. The study was conducted across 21 level III NICUs in Japan. Study period: not reported Trial registration: C000000160 (UMIN Clinical Trials Registry) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence stratified the groups based on gestational age, sex, and other factors to balance the groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was placebo‐controlled suggesting that the personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study was placebo controlled however there was no mention for how outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One infant in indomethacin group was excluded from all analyses following diagnosis with duodenal atresia. No incomplete outcomes were noted. |
| Selective reporting (reporting bias) | High risk | Protocol for original RCT was found registered prospectively at UMIN‐CTR (University hospital Medical Information Network Center) Clinical Trial Registry (C000000160). Among the stated primary outcomes, PVL, ROP and developmental impairment were not reported. |
| Other bias | Unclear risk | Treatment groups not well matched for birth weight, possibly related to the small sample size |
Ment 1985.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 24) Blinded IV Indomethacin. First 10 infants randomized to indomethacin received 0.2mg/kg IV for the 1st dose and 0.1mg/kg IV every 12 hours thereafter for a total of 5 doses. Remaining infants in the study received 0.1mg/kg per dose every 12 hours for a total of 5 doses. Control (n = 24) Equal volume IV placebo as saline |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Yale, New Haven Connecticut, USA Study period: 1 June 1983 to 28 Feb 1985 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by ordinal number of admission in blocks of 10 |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment are not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study personnel, physicians and nurses caring for study infants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ultrasound studies reviewed by blinded observers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants accounted for |
| Selective reporting (reporting bias) | Unclear risk | There is no protocol available for comparison |
| Other bias | Unclear risk | The study was terminated when statistical significance achieved, unclear if this was pre‐specified. |
Ment 1988.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 19) Blinded IV Indomethacin; initial dose of 0.1 mg/kg at 6 to 12 hours, followed by 2 doses of 0.1 mg/kg every 24 hours (3 total doses) Control (n = 17) Blinded IV placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Yale, New Haven Connecticut, USA Study period: 1 May 1985 to 31 March 1987 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By ordinal number of admission in blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment are not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study personnel, physicians and nurses caring for study infants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were reviewed by blinded observers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Echocardiography data was only available for 33 infants on day 5 due to technical difficulties. Outcomes for all randomized infants accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for comparison |
| Other bias | Low risk | Appeared free of other bias |
Ment 1994a.
| Study characteristics | ||
| Methods | Multi‐centre (3 centres) randomized controlled trial | |
| Participants |
Inclusion criteria
|
|
| Interventions |
Active intervention (n = 27) Blinded IV Indomethacin; initial dose of 0.1 mg/kg at 6 to 12 hours, followed by 2 doses of 0.1 mg/kg every 24 hours (3 total doses) Control (n = 34) Blinded IV placebo (as equal volume saline solution) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Yale New Haven Hospital, New Haven Connecticut; Women and Infants' Hopsital, Providence, RI; and Maine Medical Center, Portland, USA Study period: 5 Sept 5 1989 to 31 Aug 1992 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization procedure used |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Equal volume placebo used suggesting that care providers were blinded to the allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant is missing from analysis for oliguria without explanation. Otherwise all randomized infants accounted for. |
| Selective reporting (reporting bias) | Unclear risk | It was unclear if there were deviations from the original protocol. |
| Other bias | Low risk | Appears free of other bias. |
Ment 1994b.
| Study characteristics | ||
| Methods | Multi‐center (3 centres) randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 209) Blinded IV Indomethacin; initial dose of 0.1 mg/kg at 6 to 12 hours, followed by 2 doses of 0.1 mg/kg every 24 hours (3 total doses) Control (n = 222) Blinded IV placebo (as equal volume saline solution) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Yale New Haven Hospital, New Haven Connecticut; Women and Infants' Hopsital, Providence, RI; and Maine Medical Center, Portland, USA Study period: 5 Sept 1989‐ to 31 Aug 1992 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization procedure used |
| Allocation concealment (selection bias) | Low risk | Central allocation concealment via telephone call to pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Details of blinding not provided; however, placebo was used for control group suggesting care providers were blinded to the allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All radiologists were unaware of neonate clinical condition and randomization when evaluating ECHO. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | It was unclear if there were any deviations from the original protocol. |
| Other bias | Low risk | Appears free of other bias |
Morales‐Suarez 1994.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 40) Indomethacin Sodium Trihydrate (Indocid, *Merck Sharp and Domme), 1mg/ml solution for injection, 3 doses of 100 mcg/kg/dose every 12 hours Control (n = 40) Normal saline bolus following the same scheme as the active intervention |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Unidad de Cuidados Intensivos Neonatales, Insituto Nacional de Perinatologia, Mexico DF, Mexico Study period: not reported Trial registration: not reported Translation: translated from Spanish |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not provided |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors report trial is double blinded however do not further specify blinding efforts. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors report study was double blinded, but do not explicitly state that outcome assessors are blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data noted |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable. Unclear if any deviations from protocol exist. |
| Other bias | Low risk | No additional sources of bias were noted |
Rennie 1986.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
|
|
| Interventions |
Active intervention ( n =24) Blinded IV Indomethacin 0.2mg/kg IV. 3 doses were given at 24‐hour intervals (unless treatment stopped by care team). Control (n = 26) Identical volume saline as placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Liverpool regional NICU, UK Study period: May 1984 to June 1985 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not mention if allocation of treatment groups was randomized. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study used placebo and all personnel involved in care were blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study used placebo and all personnel involved in care were blinded to the group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcomes noted |
| Selective reporting (reporting bias) | Unclear risk | Could not judge if there were any deviations in protocol. |
| Other bias | Low risk | Appeared free of other bias. |
Sangtawesin 2006.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 22) Oral ibuprofen solution: 3 doses of ibuprofen dosed at 10mg/kg/dose via orogastric tube followed by 0.5mL distilled water. 2nd and 3rd dose were given at 24 and 48 hours after the first dose. Control ( n =20) Oral placebo that was an orange starch solution that resembled ibuprofen |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Queen Sirikit National Institute of Child Health, Thailand Study period: July 2003 to April 2004 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization method used |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo prepared by pharmacist to look like treatment, personnel blinded of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single assessor blinded to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants accounted for in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. Unclear if any deviations from protocol exist. |
| Other bias | Low risk | Appears free of other bias |
Schmidt 2001.
| Study characteristics | ||
| Methods | Multi‐center (32 centres) randomized double‐blind control trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 574) Blinded IV Indomethacin at 0.1mg/kg/dose every 24 hours for a total of 3 doses Control (n = 569) Equal volume of blinded IV placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: The primary study location was McMaster University, Hamilton, Canada. The study was conducted across 32 centres in Canada, Australia, New Zealand, Hong Kong and the USA Study period: January 1996 to March 1998 Trial registration: NCT00009646 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation by computer random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Allocation was completed by an offsite statistician, and known only to the onsite pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All syringes were partially masked with tape to ensure indomethacin and placebo vials appeared identical. Except for data monitoring committee and study pharmacists, no one involved in the study or in care/follow‐up of infants were aware of treatment group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Except for data monitoring committee and study pharmacists, no one involved in the study or in care/follow‐up of infants were aware of treatment group assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 children were lost to follow‐up in the indomethacin group (1%), and 7 children were lost to follow‐up in the control group (1.2%). All randomized infants accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Study was registered with ClinicalTrials.gov (NCT00009646) retrospectively. Study was completed from 1996 to 1998 and the study was registered in 2001. Unclear if any deviations from original protocol exist. |
| Other bias | Low risk | Appears free of other bias. |
Setzer Bandstra 1988.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 99) IV Indomethacin reconstituted with distilled water to yield 1 mg/mL indomethacin. First dose (0.2mL/kg, i.e. 0.2 mg/kg) given over 15 seconds within 12 hours of birth. Second and third doses (0.1 mL/kg, i.e. 0.1 mg/kg each) given at 12‐hour intervals thereafter. Control (n = 100) Blinded IV placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: University of Miami, USA Study period: February 1983 to June 1985 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was effected by drawing consecutive pre coded envelopes |
| Allocation concealment (selection bias) | Low risk | Patients allocated uses pre coded envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical vials of indomethacin and placebo were prepared by Merck Sharp and Dohme. Investigators unaware of group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research personnel unaware of infant treatment assignment reviewed maternal and neonatal records. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | It was unclear if there were deviations from the original protocol. |
| Other bias | Low risk | none noted |
Supapannachart 1999.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 15) IV Indomethacin 0.2mg/kg initial dose, followed by two doses of 0.1mg/kg each every 12 hours Control (n = 15) IV placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Ramathibodi Hospital, Bangkok, Thailand Study period: 1 April 1994 to 31 May1 1995 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method unspecified. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope technique used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All personnel were blinded to group, identical placebo was administered to control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All personnel were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable. Unclear if any deviations to protocol exist. |
| Other bias | Low risk | Appears free of other bias. |
Van Overmeire 2004.
| Study characteristics | ||
| Methods | Multi‐centre (7 centres) randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 205) IV Ibuprofen lysine; initial dose of 10 mg/kg within the first 6 hours of life, followed by two doses of 5 mg/kg after 24 hours and 48 hours Control (n = 210) IV placebo (normal saline) |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: the primary study location was Antwerp University Hospital, Edegem, Belgium. The study was conducted across 7 centres in Belgium Study period: 1 Feb 1 1999 to 30 Sept 2001 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done independently by the chief pharmacist at each hospital in a one‐to‐one ratio between ibuprofen and placebo, in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Details of allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Attending and consulting physicians, nurses, study collaborators, and parents were unaware of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Attending and consulting physicians, nurses, study collaborators, and parents were unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants randomized accounted for in analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for comparison |
| Other bias | Low risk | Appears free of other bias. |
Vincer 1987.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
|
|
| Interventions |
Active intervention (n = 15) IV Indomethacin 0.2mg/kg/dose; 3 doses given at 12, 24 and 36 hours after birth Control (n = 15) Identical volume of IV placebo |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: Dalhousie University, Halifax, Canada Study period: not reported Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The eligible infants were enrolled to each group in pairs, the first of each pair was randomly assigned to receive either indomethacin or placebo and the second infant in each pair received the alternate treatment |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Equal volume saline to indomethacin provided, all investigators were blinded until completion of study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators were blinded to treatment allocation until study completion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants accounted for in the primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Unable to judge as protocol was not available |
| Other bias | Low risk | Appeared free of other bias. |
Vogtmann 1988.
| Study characteristics | ||
| Methods | Single‐centre randomized controlled trial | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Active intervention (n = 19) Oral Indomethacin 0.2 mg/kg/day from days 3 to 5 Control (n = 22) Standard of care |
|
| Outcomes | Relevant outcomes for this study included
|
|
| Notes |
Primary study location: German Democratic Republic University Hospital, East Germany Study period: not reported (duration 16 months) Trial registration: not reported Translation: article translated from German |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned by random draw |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo was used, and personnel were not blinded to experimental group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Infants who died before day 8 were removed from the study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for comparison |
| Other bias | Low risk | No other obvious sources of bias identified |
BUN: blood urea nitrogen; BW: birth weight; CLD: chronic lung disease; ICU: intensive care unit; GA: gestational age; GMH: germinal matrix haemorrhage;IV: intravenous; IVH: intraventricular haemorrhage; NEC: necrotizing enterocolitis; NICU: intensive care unit; NSAID: non‐steroidal anti‐inflammatory drugs ; PDA: patent ductus arteriosus;PVH: periventricular‐intraventricular haemorrhage; PVL: periventricular leukomalacia; RCT: randomized controlled trial; RDS: respiratory distress syndrome; ROP: retinopathy of prematurity; SD:standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alfaleh 2008 | Wrong outcomes |
| Barrington 1986 | Commentary |
| Cotts 2009 | Wrong patient population |
| Domanico 1994 | Abstract only |
| Gregoire 2004 | Wrong outcomes |
| Gutierrez 1987 | Abstract only |
| Hammerman 1986 | Wrong patient population |
| Hammerman 2005 | Commentary |
| Harma 2018 | Wrong outcomes |
| Kääpä 1985 | Wrong patient population |
| Liebowitz 2017 | Wrong study design |
| Mahony 1982 | Wrong patient population |
| McGuire 2002 | commentary |
| Meau‐Petit 2005 | Conference abstract of included study |
| Ment 1987 | Conference abstract of included study |
| Ment 1998 | Commentary |
| Ment 1999 | Wrong outcomes |
| Morales‐Suarez 1992 | Conference abstract of included study |
| Naulaers 2005 | Wrong outcomes |
| Pleacher 2004 | Wrong outcomes |
| Puckett 1985 | Abstract only |
| Roze 2003 | Conference abstract of included study |
| Rubaltelli 1998 | Wrong comparator |
| Schmidt 2002 | commentary |
| Schmidt 2011 | Wrong comparator |
| Tyson 2002 | Commentary |
| Valls‐i‐Soler 1999 | Wrong comparator |
| van Overmeire 2002 | Conference abstract of included study |
| Varvarigou 1996 | Wrong study design |
| Vohr 1999 | Wrong outcomes |
| Zarkesh 2013 | Abstract only |
Characteristics of studies awaiting classification [ordered by study ID]
Akbari Asbagh 2015.
| Methods | Single‐centre randomized controlled trial |
| Participants |
Inclusion criteria
|
| Interventions |
Active intervention (n = 16) Oral acetaminophen for a period of two days starting during first 24 hours of life Control (n = 16) No placebo |
| Outcomes | Primary outcome: PDA closure |
| Notes |
Primary study location: Vali‐Asr Hospital, Tehran Study Period: March 2012 to March 2013 The article is in Persian. We contacted the primary author for further information on outcome data and we are awaiting a response |
Kalani 2016.
| Methods | Single‐centre 3‐arm study |
| Participants |
Inclusion criteria
|
| Interventions |
Active intervention 1 (n = 31) Oral ibuprofen 10, 5, 5 mg/kg every 24 hours Active intervention 2 (n = 31) Oral indomethacin 0.2 mL/kg daily for 3 days Control (n = 31) Standard of care |
| Outcomes | Relevant outcomes include
|
| Notes |
Primary study location: Akbar‐Abadi Hospital (affiliated with Iran University of Medical Sciences, Theran, Iran) Study period: 2013 to 2014 The methods section suggests that it is a retrospective study, and we were unable to establish contact with the primary author to clarify this discrepancy |
Seok 1998.
| Methods | Single‐centre randomized controlled trial |
| Participants |
Inclusion criteria
|
| Interventions |
Active intervention (n = 23) Indomethacin 0.2 mg/kg initial dose followed by 2 doses of 0.1 mg/kg at 24‐hour intervals. 15 participants received IV formulation and 8 received oral formulation Control (n = 23) No placebo |
| Outcomes | Primary outcome: germinal matrix or intraventricular haemorrhage |
| Notes |
Primary study location: Il Sin Christian Hospital, Pusan, Korea Study Period: August 1995 to June 1997 The article is in Korean. We are awaiting translation of the article from Korean to English |
GA: gestational age;GI: gastrointestinal; IVH: intraventricular haemorrhage; NEC: necrotizing enterocolitis; PDA: patent ductus arteriosus.
Characteristics of ongoing studies [ordered by study ID]
NCT03641209.
| Study name | Extremely low gestational age infants' Paracetamol Study (Paras) |
| Methods | Randomized, placebo‐controlled, double‐blind, phase 2, single‐centre clinical trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome: postnatal age of the observed closure of ductus arteriosus |
| Starting date | 3 September 2018 |
| Contact information | Principal Investigator: Outi Aikio, MD, PhD; Department of Pediatrics, Oulu University Hospital, Oulu, Finland, 90014 |
| Notes | Estimated enrolment: 40 infants Estimated primary completion date: 1 September 2022 |
NCT04459117.
| Study name | Prophylactic treatment of the ductus arteriosus in preterm infants by acetaminophen (TREOCAPA) |
| Methods | Phase II/III European multicentre randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention arm: acetaminophen In the 27 to 28 weeks gestational age group, the dosage is 2 mL/kg loading dose within 12 hours after birth followed by 0.75 mL/kg/ 6 hours during 5 days (total = 20 doses). In the 23 to 26 weeks gestational age group, the dosage will be minimum effective dose of acetaminophen to close the ductus arteriosus before or at day 7, found during the phase II. Contol arm: placebo (0.9% NaCl) |
| Outcomes | Primary outcome measure: closure of ductus arteriosus |
| Starting date | 29 October, 2020 |
| Contact information | Jean‐Christophe Rozé, Institut National de la Santé Et de la Recherche Médicale, France(jean-christophe.roze@inserm.fr) |
| Notes | Estimated enrolment: 824 infants Estimated primary completion date: April 2023 |
NaCl: sodium chloride.
Differences between protocol and review
2021
We made the following changes to the published protocol.
1. Statistical software for analysis: in the protocol we had mentioned that “We will undertake all analyses (both pairwise meta‐analyses and NMA) using the R (R Core Team 2020) package gemtc on the MetaInsight application, developed by the Cochrane Complex Review Support Unit (CRSU)”. However, the MetaInsight application was unable to generate all the pre‐defined statistical outputs such as rankograms and comparison‐adjusted forest plots. Therefore, we used the GEMTC GUI interface (van Valkenhoef 2012) which also uses the same R package gemtc to run all the analyses.
2. Presentation of relative treatment effects: in the protocol we had mentioned that the relative treatment effects “will be summarized in forest plots displaying the results from pairwise, indirect and network (combining direct and indirect) analyses”. However, the R package gemtc that was used to conduct the Bayesian random effects meta‐analysis only provided forest plot outputs for direct and network estimates. Hence, forest plots for indirect estimates were not presented in the results.
3. Heterogeneity priors for the Bayesian NMA: prior distributions for the relative effect estimates were determined heuristically based on the following: N(0, (15 ⋅ S )2), where N denotes normal distribution and S denotes the outcome scale. The outcome scale is meant to represent an unreasonably large deviation on the scale of measurement which was determined heuristically based on available data. The heterogeneity priors for the primary analyses of each of the 11 outcomes are presented in Table 13.
12. Heterogeneity priors for outcomes.
| Outcome | Heterogeneity Prior |
| Severe intraventricular haemorrhage (IVH) | standard deviation ~ uniform (0, 2.0513) |
| Mortality | standard deviation ~ uniform (0, 1.203973) |
| Receipt of pharmacotherapy for symptomatic patent cuctus arteriosus (PDA | )standard deviation ~ uniform (0, 2.944439) |
| Surgical or interventional PDA closure | standard deviation ~ uniform (0, 2.549911) |
| Necrotizing enterocolitis (NEC) | standard deviation ~ uniform (0, 1.669502) |
| Gastrointestinal perforation | standard deviation ~ uniform (0, 1.609438) |
| Chronic lung disease (CLD) | standard deviation ~ uniform (0, 1.532477) |
| Oliguria | standard deviation ~ uniform (0, 1.803594) |
| IVH of any grade | standard deviation ~ uniform (0, 1.329136) |
| Periventricular leukomalacia (PL) | standard deviation ~ uniform (0, 1.149906) |
| Cerebral palsy (CP) | standard deviation ~ uniform (0, 1.299283) |
Prior distributions for the relative effects were determined heuristically based on the following: N(0, (15 ⋅ S )2), where N denotes normal distribution and S denotes the outcome scale. The outcome scale is meant to represent an unreasonably large deviation on the scale of measurement which was determined heuristically based on available data
4. Subgroup and sensitivity analysis: none of the pre‐defined subgroup analysis (based on gestational age, birth weight or timing of initiation of prophylaxis) was possible due to lack of complete data in either subgroup in each category. Instead, we performed a post‐hoc sensitivity analysis of studies that specifically reported on infants born extremely preterm (less than 28 weeks of gestational age) and/or extremely low birth weight (less 1000 g of birth weight). We reported the sensitivity analysis results for those clinically relevant outcomes where subgroup analyses were planned a priori. Further, we did not perform the planned sensitivity analysis including only low risk of bias studies as majority of information in all the three networks (indomethacin versus placebo, ibuprofen versus placebo and acetaminophen versus placebo) was derived from studies at low risk of bias with minimal statistical heterogeneity demonstrated in the direct comparisons.
5. Outcomes for assessment of GRADE certainty of the evidence: in our protocol we had planned to include severe neurodevelopmental impairment as one of the seven outcomes for assessment of GRADE certainty of evidence. However, an NMA could not be conducted for the said outcome as this was only reported for the indomethacin versus placebo arm. Out of the listed neurodevelopmental outcomes, an NMA could be conducted for the outcome of CP. Therefore, we replaced neurodevelopmental impairment with CP as the 7th outcome for assessment of GRADE certainty of evidence.
6. Interpretation of magnitude of effect sizes for assessment of certainty of evidence: prior to assessing the certainty of evidence, the authoring team used a partially contextualized approach to define the magnitude of effect sizes for each outcome (Zeng 2021). Interpretation of effect sizes were based on a priori defined thresholds as follows: (a) For the outcome of mortality: small benefit/harm was defined as < 20 fewer or more per 1000, respectively. Moderate benefit/harm was defined as 20 to 50 fewer or more per 1000, respectively. Large benefit/harm was defined as > 50 fewer or more per 1000, respectively; (b) For all other outcomes listed in the summary of findings table: any effect < 20 fewer or more per 1000 was defined as a trivial benefit or harm. No direction of effect was specified for trivial effects. Small benefit/harm was defined as 20 to 50 fewer or more per 1000, respectively. Moderate benefit/harm was defined as 50 to 100 fewer or more per 1000, respectively. Large benefit/harm was defined as >100 fewer or more per 1000, respectively. Language for interpretation used in this column is based on the GRADE informative statements to communicate the findings of systematic reviews of interventions by Santesso 2020.
Contributions of authors
SM conceived the project, under the mentorship of BCJ and JD. SM drafted the protocol. SM, DS, AM, CEG, MCY, SK, BCJ and JD reviewed all drafts, and approved the final version of the protocol.
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
SM is the principal investigator of a Canadian Institutes of Health Research (CIHR)‐funded prospective study on the relative effectiveness and safety of pharmacotherapeutic agents for treatment of patent ductus arteriosus (PDA) in preterm infants. SM reports working as a neonatologist at a tertiary care neonatal intensive care unit in IWK Health Center (Halifax, Nova Scotia, Canada) where they attend to preterm infants diagnosed with a PDA.
CEG declares no conflict of interest.
AM declares no conflict of interest.
TD declares no conflict of interest.
DMS reports working as a Fellow (Resident PGY6) Neonatal‐Perinatal Medicine at Dalhousie University/IWK Health Center.
MCY declares no conflict of interest.
SK declares no conflict of interest.
BCJ declares no conflict of interest.
JD reports working at IWK Health as Neonatologist and therefore sometimes treat PDAs in preterm infants.
New
References
References to studies included in this review
Bada 1989 {published data only}
- Bada HS, Green RS, Pourcyrous M, Leffler CW, Korones SB, Magill HL, et al.Indomethacin reduces the risks of severe intraventricular hemorrhage. Journal of Pediatrics 1989;115(4):631-7. [DOI] [PubMed] [Google Scholar]
Bagheri 2018 {published data only}
- Bagheri M-M, Bahman-Bijari B, Torabi-Nejad M-H, Niknafs P, Mousavi H, Sabzevari F, et al.Is prophylactic parenteral paracetamol effective to diminish incidence of PDA in preterm neonates? A randomized trial. Iranian Journal of Pediatrics 2018;28(4):e11735. [Google Scholar]
Couser 1996 {published data only}
- Couser R J, Ferrara TB, Wright GB, Cabalka AK, Schilling CG, Hoekstra RE, et al.Prophylactic indomethacin therapy in the first twenty-four hours of life for the prevention of patent ductus arteriosus in preterm infants treated prophylactically with surfactant in the delivery room. Journal of Pediatrics 1996;128(5 Pt 1):631-7. [DOI] [PubMed] [Google Scholar]
- Couser RJ, Hoekstra RE, Ferrara B, Wright GB, Cabalka AK, Connet JE.Neurodevelopmental follow-up at 36months’ corrected age of preterm infants treated withprophylactic indomethacin. Archives of Pediatric and Adolescent Medicine 2000;154(6):598-602. [DOI] [PubMed] [Google Scholar]
Dani 2000 {published data only}
- Dani C, Bertini G, Reali M F, Murru P, Fabris C, Vangi V, et al.Prophylaxis of patent ductus arteriosus with ibuprofen in preterm infants. Acta Paediatrica 2000;89(11):1369-74. [DOI] [PubMed] [Google Scholar]
Dani 2005 {published data only}
- Dani C, Bertini G, Pezzati M, Poggi C, Guerrini P, Martano C, et al.Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics 2005;115(6):1529-35. [DOI] [PubMed] [Google Scholar]
De Carolis 2000 {published data only}
- De Carolis M P, Romagnoli C, Polimeni V, Piersigilli F, Zecca E, Papacci P, et al.Prophylactic ibuprofen therapy of patent ductus arteriosus in preterm infants. European Journal of Pediatrics 2000;159(5):364-8. [DOI] [PubMed] [Google Scholar]
Gournay 2004 {published data only}
- Gournay V, Roze J C, Kuster A, Daoud P, Cambonie G, Hascoet J M, et al.Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double-blind, placebo-controlled trial. Lancet 2004;364(9449):1939-44. [DOI] [PubMed] [Google Scholar]
Hanigan 1988 {published data only}
- Hanigan WC, Kennedy G, Roemisch F, Anderson R, Cusack T, Powers W.Administration of indomethacin for the prevention of periventricular-intraventricular hemorrhage in high-risk neonates. Journal of Pediatrics 1988;112(6):941-7. [DOI] [PubMed] [Google Scholar]
Harkin 2016 {published data only}
- Harkin P, Harma A, Aikio O, Valkama M, Leskinen M, Saarela T, et al.Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. Journal of Pediatrics 2016;177:72-7.e2. [DOI] [PubMed] [Google Scholar]
- Juujärvi S, Kallankari H, Pätsi P, Leskinen M, Saarela T, Hallman M, et al.Follow-up study of the early, randomised paracetamol trial to preterm infants, found no adverse reactions at the two-years corrected age. Acta Paediatrica 2019;108(3):452-8. [DOI] [PubMed] [Google Scholar]
Jannatdoust 2014 {published data only}
- Jannatdoust A, Samadi M, Yeganehdoust S, Heydarzadeh M, Alikhah H, Piri R, et al.Effects of intravenous indomethacin on reduction of symptomatic patent ductus arteriosus cases and decreasing the need for prolonged mechanical ventilation. Journal of Cardiovascular and Thoracic Research 2014;6(4):257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanmaz 2013 {published data only}
- Kanmaz G, Erdeve O, Canpolat FE, Oğuz SS, Uras N, Altug N, et al.Serum ibuprofen levels of extremely preterm infants treated prophylactically with oral ibuprofen to prevent patent ductus arteriosus. European Journal of Clinical Pharmacology 2013;69(5):1075-81. [DOI] [PubMed] [Google Scholar]
Krueger 1987 {published data only}
- Krueger E, Mellander M, Bratton D, Cotton R.Prevention of symptomatic patent ductus arteriosus with a single dose of indomethacin. Journal of Pediatrics 1987;111(5):749-54. [DOI] [PubMed] [Google Scholar]
Kumar Nair 2004 {published data only}
- Kumar Nair PA, Pai MG, Gazal HA, Da Costa DE, Al Khusaiby SM.Indomethacin prophylaxis for intraventricular hemorrhage in very low birth weight babies. Indian Pediatrics 2004;41(6):551-8. [PubMed] [Google Scholar]
Mahony 1985 {published data only}
- Mahony L, Caldwell RL, Girod D A, Hurwitz RA, Jansen RD, Lemons JA, et al.Indomethacin therapy on the first day of life in infants with very low birth weight. Journal of Pediatrics 1985;106(5):801-5. [DOI] [PubMed] [Google Scholar]
Maruyama 2012 {published data only}
- Maruyama K, Fujiu T.Effects of prophylactic indomethacin on renal and intestinal blood flows in premature infants. Pediatrics International 2012;54(4):480-5. [DOI] [PubMed] [Google Scholar]
Ment 1985 {published data only}
- Ment L R, Duncan CC, Ehrenkranz RA, Kleinman CS, Pitt BR, Taylor KJ, et al.Randomized indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight infants. Journal of Pediatrics 1985;107(6):937-43. [DOI] [PubMed] [Google Scholar]
Ment 1988 {published data only}
- Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Taylor K J, Scott DT, et al.Randomized low-dose indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates. Journal of Pediatrics 1988;112(6):948-55. [DOI] [PubMed] [Google Scholar]
Ment 1994a {published data only}
- Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr B, Allan W, et al.Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. Journal of Pediatrics 1994;124(6):951-5. [DOI] [PubMed] [Google Scholar]
Ment 1994b {published data only}
- Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR.Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics 2009;123(3):1037-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al.Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics 1994;93(4):543-50. [PubMed] [Google Scholar]
- Ment LR, Vohr B, Allan W, Westerveld M, SparrowSS, Schneider KC, et al.Outcome of children in theindomethacin intraventricular hemorrhage prevention trial. Pediatrics 2000;105:485-91. [DOI] [PubMed] [Google Scholar]
- Ment LR, Vohr B, Oh W, Scott DT, Allan WC, Westerveld M, et al.Neurodevelopmental outcome at 36 months' corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics 1996;98:714-8. [PubMed] [Google Scholar]
- Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, et al.Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. Journal of Pediatrics 2004;145(6):832-4. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, et al.School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics 2003;111(4 Pt 1):e340-6. [DOI] [PubMed] [Google Scholar]
Morales‐Suarez 1994 {published data only}
- Morales-Suarez M, De Jesus Sanchez-Gil T, Lemus-Varela L, Udaeta-Mora E.Low doses indomethacin for prevention of intraventricular hemorrhage in the preterm newborn infant with mechanical ventilation: final report of a randomized study [Estudiocomparavito de dosis baja de indometacina profilactica parahemorragia subependimaria/intraventricular en neonatospretermino con ventilation mecanica]. Boletin Medico del Hospital Infantil de Mexico 1994;51(6):389-94. [Google Scholar]
Rennie 1986 {published data only}
- Rennie J M, Doyle J, Cooke RW.Early administration of indomethacin to preterm infants. Archives of Disease in Childhood 1986;61(3):233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sangtawesin 2006 {published data only}
- Sangtawesin V, Sangtawesin C, Raksasinborisut C, Sathirakul K, Kanjanapattanakul W, Khorana M, et al.Oral ibuprofen prophylaxis for symptomatic patent ductus arteriosus of prematurity. Journal of the Medical Association of Thailand 2006;89(3):314-21. [PubMed] [Google Scholar]
Schmidt 2001 {published data only}
- Ohlsson A, Roberts RS, Schmidt B, Davis P, ModdemanD, Saigal S, et al.Male/female differences in indomethacineffects in preterm infants. Journal of Pediatrics 2005;147:860-2. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al.Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. New England Journal of Medicine 2001;344(26):1966-72. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, et al.Indomethacin prophylaxis, patent ductusarteriosus, and the risk of bronchopulmonary dysplasia:further analyses from the Trial of Indomethacin Prophylaxisin Preterms (TIPP). Journal of Pediatrics 2006;148:730-4. [DOI] [PubMed] [Google Scholar]
- Zupancic JA, Richardson DK, O’Brien BJ, Cronin CG, Schmidt B, Roberts R, et al.Retrospective economic evaluation of a controlled trial of indomethacin prophylaxis for patent ductus arteriosus in premature infants. Early Human Development 2006;82:97-103. [DOI] [PubMed] [Google Scholar]
Setzer Bandstra 1988 {published data only}
- Bandstra ES, Montalvo BM, Goldberg RN, Pacheco I, Ferrer PL, Flynn J, et al.Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics 1988;82(4):533-42. [PubMed] [Google Scholar]
- Setzer ES, Morse BM, Goldberg RN, Smith M, Bancalari E.Prophylactic indomethacin and intraventricular hemorrhagein the premature. In: Pediatric Research. Vol. 18. 1984:345A.
- Setzer ES, Torres-Arraut E, Gomez-del-Rio M, Young ML, Pacheco I, Ferrer PL, et al.Cardiopulmonary effects of prophylactic indomethacin in the very low birth weight infant. In: Pediatric Research. Vol. 18. 1984:346A.
Supapannachart 1999 {published data only}
- Supapannachart S, Khowsathit P, Patchakapati B.Indomethacin prophylaxis for patent ductus arteriosus (PDA) in infants with a birth weight of less than 1250 grams. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet 1999;82 Suppl 1:S87-92. [PubMed] [Google Scholar]
Van Overmeire 2004 {published data only}
- Van Overmeire B, Allegaert K, Casaer A, Debauche C, Decaluwé W, Jespers A, et al.Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2004;364(9449):1945-9. [DOI] [PubMed] [Google Scholar]
- Van Overmeire B, Casaer A, Allegaert K, Debauche C, Decaluwe W, Jespers A.Multicenter ibuprofen prophylaxis study (MIPS) in preterm infants: preliminary data. Pediatric Research 2002;52:825. [Google Scholar]
Vincer 1987 {published data only}
- Vincer M, Allen A, Evans J, Nwaesei C, Stinson D, Rees E, et al.Early intravenous indomethacin prolongs respiratory support in very low birth weight infants. Acta Paediatrica 1987;76(6):894-7. [DOI] [PubMed] [Google Scholar]
- Vincer M, Allen A.Does prophylactic indomethacin inVLBW (<1500 grams birth weight) infants cause cerebral palsy (CP)? Pediatric Research 1998;43:232. [Google Scholar]
Vogtmann 1988 {published data only}
- Vogtmann C, Grubbe G, Ruckhäberle KE, Böttcher H, Ockert C.Effects of early therapy with indomethacin on the manifestation of a persistent ductus arteriosus in extremely underweight premature infants [Auswirkungen einer Frühtherapie mit Indomethazin auf die Manifestation eines persistierenden Ductus arteriosus bei extrem untergewichtigen Frühgeborenen]. Monatsschrift Kinderheilkund 1988;136(9):636-9. [PubMed] [Google Scholar]
References to studies excluded from this review
Alfaleh 2008 {published data only}
- Alfaleh K, Smyth JA, Roberts RS, Solimano A, Asztalos EV, Schmidt B, Trial of Indomethacin Prophylaxis in Preterms Investigators.Prevention and 18-month outcomes of serious pulmonary hemorrhage in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. Pediatrics 2008;121(2):e233-8. [DOI] [PubMed] [Google Scholar]
Barrington 1986 {published data only}
- Barrington KJ, Finer NN, Ment LR.Indomethacin for prevention of intraventricular hemorrhage. Journal of Pediatrics 1986;109(2):396-8. [DOI] [PubMed] [Google Scholar]
Cotts 2009 {published data only}
- Cotts T.Escalating dose indomethacin for prophylactic closure of patent ductus arteriosus does not improve closure rates and is associated with increased complications. Journal of Pediatrics 2009;154(1):153. [DOI] [PubMed] [Google Scholar]
Domanico 1994 {published data only}
- Domanico RS, Waldman JD, Lester LA, McPhillips HA, Catrambone JE, Covert RF.Prophylactic indomethacin reduces the incidence of pulmonary hemorrhage and patent ductus arteriosus in surfactant-treated infants. Pediatric Research 1994;35:331A. [Google Scholar]
Gregoire 2004 {published data only}
- Gregoire N, Gualano V, Geneteau A, Millerioux L, Brault M, Mignot A, et al.Population pharmacokinetics of ibuprofen enantiomers in very premature neonates. Journal of Clinical Pharmacology 2004;44(10):1114-24. [DOI] [PubMed] [Google Scholar]
Gutierrez 1987 {published data only}
- Gutierrez NG, Lapasset M.Prophylactic indomethacin and the incidence of patent ductus arteriosus in preterm neonates. Proceedings of the 3rd Argentinian Congress of Perinatology, Buenos Aires 1987;62.
Hammerman 1986 {published data only}
- Hammerman C, Strates E, Valaitis S.The silent ductus: its precursors and its aftermath. Pediatric Cardiology 1986;7(3):121-7. [DOI] [PubMed] [Google Scholar]
Hammerman 2005 {published data only}
- Hammerman C, Kaplan M.Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Journal of Pediatrics 2005;146(5):709-10. [DOI] [PubMed] [Google Scholar]
Harma 2018 {published data only}
- Harma A, Harkin P, Leskinen M, Valkama M, Saarela T, Aikio O, et al.Does intravenous paracetamol offer neonatal brain protection? Study of hemodynamics and cerebral oxygenation in very premature infants. Neonatology 2018;113(4):417-8. [DOI: ] [Google Scholar]
Kääpä 1985 {published data only}
- Kääpä P.Early closure of ductus arteriosus with indomethacin [Der Frühverschluss des Ductus arteriosus mit Indomethazin]. Klinische Pädiatrie 1985;197(2):172. [DOI: doi: 10.1055/s-2008-1033961] [DOI] [PubMed] [Google Scholar]
Liebowitz 2017 {published data only}
- Liebowitz M, Clyman RI.Prophylactic indomethacin compared with delayed conservative management of the patent ductus arteriosus in extremely preterm infants: effects on neonatal outcomes. Journal of Pediatrics 2017;187:119-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahony 1982 {published data only}
- Mahony L, Carnero V, Brett C, Heymann MA, Clyman RI.Prophylactic indomethacin therapy for patent ductus arteriosus in very-low-birth-weight infants. New England Journal of Medicine 1982;306(9):506-10. [DOI] [PubMed] [Google Scholar]
McGuire 2002 {published data only}
- McGuire W, Fowlie PW.Treating extremely low birthweight infants with prophylactic indomethacin. Evidence for short term benefits only. BMJ 2002;324(7329):60-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meau‐Petit 2005 {published data only}
- Meau-Petit V.Prophylactic ibuprofen vs placebo in very premature infants: a randomised, double blind, placebo-controlled trial. Archives de Pédiatrie 2005;12:605-8. [Google Scholar]
Ment 1987 {published data only}
- Ment LR.Randomized low-dose indomethacin trial for the prevention of intraventricular hemorrhage in very low birth weight neonates. Annals of Neurology 1987;22(3):406-7. [DOI] [PubMed] [Google Scholar]
Ment 1998 {published data only}
- Ment LR, Westerveld M, Makuch R, Vohr B, Allan WC.Cognitive outcome at 4 1/2 years of very low birth weight infants enrolled in the multicenter indomethacin intraventricular hemorrhage prevention trial. Pediatrics 1998;102(1 Pt 1):159-60. [DOI] [PubMed] [Google Scholar]
Ment 1999 {published data only}
- Ment LR, Vohr B, Allan W, Westerveld M, Katz KH, Schneider KC, et al.The etiology and outcome of cerebral ventriculomegaly at term in very low birth weight preterm infants. Pediatrics 1999;104(2 Pt 1):243-8. [DOI] [PubMed] [Google Scholar]
Morales‐Suarez 1992 {published data only}
- Morales-Suarez M, Lemus-Varela L, Udaeta-Mora E, Cardiel-Marmolejo L, Rodríguez-Balderrama I, Liz-Cedillo RE.Indomethacin in the prevention of subependymal-intraventricular hemorrhage in preterm newborns with conventional mechanical ventilation [Indometacina en la prevención de la hemorragia subependimaria-intraventricular del recién nacido pretérmino con ventilación mecánica convencional]. Boletín Médico del Hospital Infantil de México 1992;49(4):217-24. [PubMed] [Google Scholar]
Naulaers 2005 {published data only}
- Naulaers G, Delanghe G, Allegaert K, Debeer A, Cossey V, Vanhole C, et al.Ibuprofen and cerebral oxygenation and circulation. Archives of Disease in Childhood. Fetal Neonatal Edition 2005;90(1):F75-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pleacher 2004 {published data only}
- Pleacher MD, Vohr BR, Katz KH, Ment LR, Allan WC.An evidence-based approach to predicting low IQ in very preterm infants from the neurological examination: outcome data from the indomethacin Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics 2004;113(2):416-9. [DOI] [PubMed] [Google Scholar]
Puckett 1985 {published data only}
- Puckett CG, Cox MA, Haskins KS, Fisher DJ.Prophylactic indomethacin (I) for the prevention of patent ductus arteriosus (PDA). Pediatric Research 1985;19:358. [Google Scholar]
Roze 2003 {published data only}
- Roze JC, Gournay V, Daoud P, Cambonie G, Chamboux C, Hascoet JM, et al.Multicenter double blind randomized placebo-controlled study comparing prophylactic versus curative ibuprofen treatment in premature infants less than 28 weeks of gestation. Pediatric Research 2003;53:386A. [Google Scholar]
Rubaltelli 1998 {published data only}
- Rubaltelli F, Bertini G, Reali MF, Vangi V, Dani C.Does early closure of PDA with ibuprofen reduce the severity of RDS in premature infants? Pediatric Research 1998;43:296A. [Google Scholar]
Schmidt 2002 {published data only}
- Schmidt B, Wright LL, Davis P, Solimano A, Roberts RS, Indomethacin Prophylaxis in Preterms Investigators.Ibuprofen prophylaxis in preterm neonates. Lancet 2002;360(9331):492. [DOI] [PubMed] [Google Scholar]
Schmidt 2011 {published data only}
- Schmidt B, Seshia M, Shankaran S, Mildenhall L, Tyson J, Lui K, et al, Trial of Indomethacin Prophylaxis in Preterms Investigators.Effects of prophylactic indomethacin in extremely low-birth-weight infants with and without adequate exposure to antenatal corticosteroids. Archives of Pediatrics and Adolescent Medicine 2011;165(7):642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyson 2002 {published data only}
- Tyson JE.Does indomethacin prophylaxis benefit extremely low birth weight infants? Results of a placebo-controlled multicenter trial. Pediatric Research 2002;51(1):1. [Google Scholar]
Valls‐i‐Soler 1999 {published data only}
- Valls-i-Soler A, Lopez-de-Heredia J, Roman L, Fernandez-Ruanova MB.Prophylactic indomethacin (INDO) in very low birth weight (VLBW) infants with RDS. A pilot study on the effects of a slow infusion rate. Pediatric Research 1999;45(6):901. [Google Scholar]
van Overmeire 2002 {published data only}
- Overmeire B, Casaer A, Allegaert K, Debauche C, Decaluwe W, Jespers A, et al.Multicenter randomized double-blind placebo-controlled trial of ibuprofen prophylaxis in very preterm infants. Pediatric Research 2002;51(4):379A. [Google Scholar]
Varvarigou 1996 {published data only}
- Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A, Aranda JV.Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA 1996;275(7):539-44. [PMID: ] [PubMed] [Google Scholar]
Vohr 1999 {published data only}
- Vohr B, Allan WC, Scott DT, Katz KH, Schneider KC, Makuch RW, et al.Early-onset intraventricular hemorrhage in preterm neonates: incidence of neurodevelopmental handicap. Seminars in Perinatology 1999;23(3):212-7. [DOI] [PubMed] [Google Scholar]
Zarkesh 2013 {published data only}
- Zarkesh MR, Nili F, Akbari Asbagh P, Nayeri FS, Naeem A.Prophylactic treatment with oral paracetamol for patent ductus arteriosus in preterm infants admitted in Nicu Vali-Asr Hospital In 2012. Iranian Journal Of Pediatrics 2013;23(Suppl 1):54-5. [Google Scholar]
References to studies awaiting assessment
Akbari Asbagh 2015 {published data only}
- Akbari Asbagh P, Zarkesh MR, Nili F, Nayeri FS, Tofighi Naeem A.Prophylactic teatment with oral paracetamol for patent ductus arteriosus in preterm infants: a randomized clinical trial. Tehran University Medical Journal 2015;73(2):86-92. [Google Scholar]
Kalani 2016 {published data only}
- Kalani M, Shariat M, Khalesi N, Farahani Z, Ahmadi S.A comparison of early ibuprofen and indomethacin administration to prevent intraventricular hemorrhage among preterm infants. Acta Medica Iranica 2016;54(12):788-92. [PubMed] [Google Scholar]
Seok 1998 {published data only}
- Seok EJ, Kim EJ, Jeon SS, Seo SS.Effect of indomethacin therapy on prevention of intraventricular hemorrhage in very low birth weight infant. Journal of the Korean Society of Neonatal Science 1998;5(1):27-34. [Google Scholar]
References to ongoing studies
NCT03641209 {published data only}
- Aikio O.Extremely low gestatonal age infants' paracetamol study (Paras) [Extremely low gestational age infants' paracetamol study: a randomized trial]. https://clinicaltrials.gov/ct2/show/NCT03641209 (first received 21 August 2018).
NCT04459117 {published data only}
- Rozé JC.Prophylactic treatment of the ductus arteriosus in preterm Infants by acetaminophen (TREOCAPA). https://clinicaltrials.gov/ct2/show/NCT04459117 (first received 7 July 2020).
Additional references
Baik 2015
- Baik N, Urlesberger B, Schwaberger B, Schmölzer GM, Avian A, Pichler G.Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Archives of Disease in Childhood: Fetal and Neonatal Edition 2015;100(5):F422-7. [DOI] [PubMed] [Google Scholar]
Baker 2002
- Baker SG, Kramer BS.The transitive fallacy for randomized trials: if A bests B and B bests C in separate trials, is A better than C? BMC Medical Research Methodology 2002;2:13. [DOI: 10.1186/1471-2288-2-13] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballabh 2010
- Ballabh P.Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric Research 2010;67(1):1-8. [DOI: 10.1203/PDR.0b013e3181c1b176] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 2013
- Bauer AZ, Kriebel D.Prenatal and perinatal analgesic exposure and autism: an ecological link. Environmental Health 2013;12:41. [DOI: 10.1186/1476-069X-12-41] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 1993
- Steiner S.Bayley Scales of Infant Development. 2nd edition. San Antonio, TX: The Psychological Corporation, 1993. [Google Scholar]
Bayley 2005
- Bayley N.Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: The Psychological Corporation, 2005. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al.Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benitz 2016
- Benitz WE, Committee on Fetus and Newborn, American Academy of Pediatrics.Patent ductus arteriosus in preterm infants. Pediatrics 2016;137(1):epub. [DOI: 10.1542/peds.2015-3730] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bisaglia 2002
- Bisaglia M, Venezia V, Piccioli P, Stanzione S, Porcile C, Russo C, et al.Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochemistry International 2002;41(1):43-54. [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2018
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al, GRADE Working Group.Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. [DOI: 10.1016/j.jclinepi.2017.10.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2021
- Brignardello-Petersen R, Guyatt GH, Mustafa RA, Chu DK, Hultcrantz M, Schünemann HJ, et al.GRADE guidelines 33: Addressing imprecision in a network meta-analysis. Journal of Clinical Epidemiology 2021;139:49-56. [DOI: 10.1016/j.jclinepi.2021.07.011] [DOI] [PubMed] [Google Scholar]
Brown 1979
- Brown ER.Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. Journal of Pediatrics 1979;95(5 Pt 2):865-6. [DOI: 10.1016/s0022-3476(79)80454-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chung 2005
- Chung M-Y, Fang P-C, Chung C-H, Huang C-B, Ou Yang M-H, Chen C-C.Risk factors for hemodynamically-unrelated cystic periventricular leukomalacia in very low birth weight premature infants. Journal of the Formosan Medical Association 2005;104(8):571-7. [PMID: ] [PubMed] [Google Scholar]
Cimatti 2020
- Cimatti AG, Martini S, Galletti S, Vitali F, Aceti A, Frabboni G, et al.Cerebral oxygenation and autoregulation in very preterm infants developing IVH during the transitional period: a pilot study. Frontiers in Pediatrics 2020;8:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G.Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI: 10.7326/0003-4819-159-2-201307160-00008] [PMID: 23856683] [DOI] [PubMed] [Google Scholar]
Clyman 2012
- Clyman RI, Couto J, Murphy GM.Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Seminars in Perinatology 2012;36(2):123-9. [DOI: 10.1053/j.semperi.2011.09.022] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coombs 1991
- Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS.Gut blood flow velocities in the newborn: effects of patent ductus arteriosus and parenteral indomethacin. Archives of Disease in Childhood 1991;66(10):1261. [DOI: 10.1136/adc.65.10_spec_no.1067] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costantini 2020
- Costantini I, Paul E, Caldwell DM, López-López JA, Pearson RM.Protocol for a systematic review and network meta-analysis of randomised controlled trials examining the effectiveness of early parenting interventions in preventing internalising problems in children and adolescents. Systematic Reviews 2020;9(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Couser 2000
- Couser RJ, Hoekstra RE, Ferrara B, Wright GB, Cabalka AK, Connet JE.Neurodevelopmental follow-up at 36months’ corrected age of preterm infants treated withprophylactic indomethacin. Archives of Pediatric and Adolescent Medicine 2000;154(6):598-602. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence.Version accessed 15 September 2018. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
de Vries 1992
- Vries LS, Eken P, Dubowitz LM.The spectrum of leukomalacia using cranial ultrasound. Behavioural Brain Research 1992;49(1):1-6. [DOI: 10.1016/s0166-4328(05)80189-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell D M, Ades AE.Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [DOI: 10.1002/sim.3767] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dias 2013
- Dias S, Sutton AJ, Ades AE, Welton NJ.Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Medical Decision Making: an International Journal of the Society for Medical Decision Making 2013;33(5):607-17. [DOI: 10.1177/0272989X12458724] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dice 2007
- Dice JE, Bhatia J.Patent ductus arteriosus: an overview. Journal of Pediatric Pharmacology and Therapeutics 2007;12(3):138-46. [DOI: 10.5863/1551-6776-12.3.138] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dollberg 2005
- Dollberg S, Lusky A, Reichman B.Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. Journal of Pediatric Gastroenterology and Nutrition 2005;40(2):184-8. [DOI: 10.1097/00005176-200502000-00019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Donegan 2010
- Donegan S, Williamson P, Gamble C, Tudur-Smith C.Indirect comparisons: a review of reporting and methodological quality. PLOS One 2010;5(11):e11054. [DOI: 10.1371/journal.pone.0011054] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drougia 2007
- Drougia A, Giapros V, Krallis N, Theocharis P, Nikaki A, Tzoufi M, et al.Incidence and risk factors for cerebral palsy in infants with perinatal problems: a 15-year review. Early Human Development 2007;83(8):541-7. [DOI: 10.1016/j.earlhumdev.2006.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ehrenkranz 2005
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al, National Institutes of Child Health and Human Development Neonatal Research Network.Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353-60. [DOI: 10.1542/peds.2005-0249] [PMID: 16322158] [DOI] [PubMed] [Google Scholar]
Fowlie 2010
- Fowlie PW, Davis PG, McGuire W.Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 7. Art. No: CD000174. [DOI: 10.1002/14651858.CD000174.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 1976
- Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE.Pharmacologic closure of patent ductus arteriosus in the premature infant. New England Journal of Medicine 1976;295(10):526-9. [DOI: 10.1056/NEJM197609022951003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gelman 1992
- Gelman A, Rubin DB.Inference from iterative simulation using multiple sequences. Statistical Science 1992;7(4):457-72. [DOI: 10.1214/ss/1177011136] [DOI] [Google Scholar]
Gournay 2011
- Gournay V.The ductus arteriosus: physiology, regulation, and functional and congenital anomalies. Archives of Cardiovascular Diseases 2011;104(11):578-85. [DOI: 10.1016/j.acvd.2010.06.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Grèen 1989
- Grèen K, Drvota V, Vesterqvist O.Pronounced reduction of in vivo prostacyclin synthesis in humans by acetaminophen (paracetamol). Prostaglandins 1989;37(3):311-5. [DOI: 10.1016/0090-6980(89)90001-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R.The Abilities of Babies: a Study of Mental Measurement. London: University of London Press, 1954. [Google Scholar]
Griffiths 1970
- Griffiths R.The Abilities of Young Children: a Comprehensive System of Mental Measurement for the First Eight Years. London: Child Development Research Center, 1970. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group.GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI: 10.1136/bmj.39489.470347.AD] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamrick 2020
- Hamrick SE, Sallmon H, Rose AT, Porras D, Shelton EL, Reese J, Hansmann G.Patent ductus arteriosus of the preterm infant. Pediatrics 2020;146(5):e20201209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Härkin 2016
- Härkin P, Härmä A, Aikio O, Valkama M, Leskinen M, Saarela T, et al.Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. Journal of Pediatrics 2016;177:72-7.e2. [DOI] [PubMed] [Google Scholar]
Härmä 2020
- Härmä A, Aikio O, Härkin P, Leskinen M, Valkama M, Saarela T, et al.Subgroup analysis of the early paracetamol trial to preterm infants found haemodynamic changes and improved oxygenation. Early Human Development 2020;145:105042. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors).Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG.Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2015
- Jain A, Shah PS.Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatrics 2015;169(9):863-72. [DOI: 10.1001/jamapediatrics.2015.0987] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ji 2020
- Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, et al.Association of cord plasma biomarkers of In utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry 2020;77(2):180-9. [DOI: 10.1001/jamapsychiatry.2019.3259] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Juujärvi 2019
- Juujärvi S, Kallankari H, Pätsi P, Leskinen M, Saarela T, Hallman M, et al.Follow-up study of the early, randomised paracetamol trial to preterm infants, found no adverse reactions at the two-years corrected age. Acta Paediatrica, International Journal of Paediatrics 2019;108(3):452-8. [DOI] [PubMed] [Google Scholar]
Lambert 2005
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR.How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Statistics in Medicine 2005;24(15):2401-28. [DOI: 10.1002/sim.2112] [PMID: ] [DOI] [PubMed] [Google Scholar]
Le 2015
- Le J, Gales MA, Gales BJ.Acetaminophen for patent ductus arteriosus. Annals of Pharmacotherapy 2015;49(2):241-6. [DOI: 10.1177/1060028014557564] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lu 2004
- Lu G, Ades AE.Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23(20):3105-24. [DOI: 10.1002/sim.1875] [PMID: ] [DOI] [PubMed] [Google Scholar]
Luu 2009
- Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR.Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics 2009;123(3):1037-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ment 1992
- Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA.Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke 1992;23(8):1132-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ment 1996
- Ment L R, Vohr B, Oh W, Scott D T, Allan W C, Westerveld M, et al.Neurodevelopmental outcome at 36 months' corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics 1996;98:714-8. [PubMed] [Google Scholar]
Ment 2000
- Ment LR, Vohr B, Allan W, Westerveld M, Sparrow SS, Schneider KC, et al.Outcome of children in theindomethacin intraventricular hemorrhage prevention trial. Pediatrics 2000;105:485-91. [DOI] [PubMed] [Google Scholar]
Ment 2004
- Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, et al.Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. Journal of Pediatrics 2004;145(6):832-4. [DOI] [PubMed] [Google Scholar]
Meyer 1991
- Meyer CL, Payne NR, Roback SA.Spontaneous, isolated intestinal perforations in neonates with birth weight less than 1,000 g not associated with necrotizing enterocolitis. Journal of Pediatric Surgery 1991;26(6):714-7. [DOI: 10.1016/0022-3468(91)90017-n] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mitra 2018
- Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki A, et al.Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA 2018;319(12):1221-38. [DOI: 10.1001/jama.2018.1896] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ohlsson 1993
- Ohlsson A, Bottu J, Govan J, Ryan ML, Fong K, Myhr T.Effect of indomethacin on cerebral blood flow velocities in very low birth weight neonates with a patent ductus arteriosus. Developmental Pharmacology and Therapeutics 1993;20(1-2):100-6. [DOI: 10.1159/000457546] [PMID: 7924757] [DOI] [PubMed] [Google Scholar]
Ohlsson 2005
- Ohlsson A, Roberts RS, Schmidt B, Davis P, ModdemanD, Saigal S, et al.Male/female differences in indomethacin effects in preterm infants. Journal of Pediatrics 2005;147:860-2. [DOI] [PubMed] [Google Scholar]
Ohlsson 2020a
- Ohlsson A, Walia R, Shah SS.Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD003481. [DOI: 10.1002/14651858.CD003481.pub8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohlsson 2020b
- Ohlsson A, Shah PS.Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD010061. [DOI: 10.1002/14651858.CD010061.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohlsson 2020c
- Ohlsson A, Shah SS.Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD004213. [DOI: 10.1002/14651858.CD004213.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Owen 2019
- Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A.MetaInsight: an interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Research Synthesis Methods 2019;10(4):569-81. [DOI: 10.1002/jrsm.1373] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2016
- Palmer T, Sterne J, editors.Meta-Analysis in Stata. In: An Updated Collection From the Stata Journal. College Station (TX): Stata Press, 2016. [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H.Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al.A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI: 10.1136/bmj.g5630] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pumberger 2002
- Pumberger W, Mayr M, Kohlhauser C, Weninger M.Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. Journal of the American College of Surgeons 2002;195(6):796-803. [DOI: 10.1016/s1072-7515(02)01344-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
R Core Team 2020 [Computer program]
- R Foundation for Statistical Computing R: A language and environment for statistical computing.Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at www.R-project.org.
Reese 2017
- Reese J, Shelton EL, Slaughter JC, McNamara PJ.Prophylactic indomethacin revisited. Journal of Pediatrics 2017;186:11-4.e1. [DOI: 10.1016/j.jpeds.2017.03.036] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2019
- Ryan M, Lacaze-Masmonteil T, Mohammad K.Neuroprotection from acute brain injury in preterm infants. Paediatrics and Child Health 2019;24(4):276-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP.Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI: 10.1016/j.jclinepi.2010.03.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sangtawesin 2008
- Sangtawesin C, Sangtawesin V, Lertsutthiwong W, Kanjanapattanakul W, Khorana M, Ayudhaya JK.Prophylaxis of symptomatic patent ductus arteriosus with oral ibuprofen in very low birth weight infants. Journal of the Medical Association of Thailand 2008;91(Suppl 3):S28-34. [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al, GRADE Working Group.GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Schmidt 2006
- Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, et al.Indomethacin prophylaxis, patent ductusarteriosus, and the risk of bronchopulmonary dysplasia:further analyses from the Trial of Indomethacin Prophylaxisin Preterms (TIPP). Journal of Pediatrics 2006;148:730-4. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brozek J, Guyatt G, Oxman A, editors.Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Setzer 1984a
- Setzer ES, Morse BM, Goldberg RN, Smith M, Bancalari E.Prophylactic indomethacin and intraventricular hemorrhage in the premature. In: Pediatric Research. Vol. 18. 1984:345.
Setzer 1984b
- Setzer ES, Torres-Arraut E, Gomez-del-Rio M, Young ML, Pacheco I, Ferrer PL, et al.Cardiopulmonary effects of prophylactic indomethacin in the very low birth weight infant. In: Pediatric Research. Vol. 18. 1984:346.
Seyberth 1983
- Seyberth HW, Rascher W, Hackenthal R, Wille L.Effect of prolonged indomethacin therapy on renal function and selected vasoactive hormones in very-low-birth-weight infants with symptomatic patent ductus arteriosus. Journal of Pediatrics 1983;103(6):979-84. [DOI: 10.1016/s0022-3476(83)80736-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stavel 2017
- Stavel M, Wong J, Cieslak Z, Sherlock R, Claveau M, Shah PS.Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. Journal of Perinatology: Official Journal of the California Perinatal Association 2017;37(2):188-93. [DOI: 10.1038/jp.2016.196] [PMID: ] [DOI] [PubMed] [Google Scholar]
The Canadian Neonatal Network 2019
- The Canadian Neonatal Network.CNN 2018 Annual Report. Available at canadianneonatalnetwork.org/portal/Default.aspx (accessed 15 December 2020).
Van Overmeire 2002
- Van Overmeire B, Casaer A, Allegaert K, Debauche C, Decaluwe W, Jespers A.Multicenter ibuprofen prophylaxis study (MIPS) in preterm infants: preliminary data. Pediatric Research 2002;52:825. [Google Scholar]
van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ.Automating network meta-analysis. Research Synthesis Methods 2012;3(4):285-99. [DOI: 10.1002/jrsm.1054] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vergeade 2016
- Vergeade A, Bertram CC, Bikineyeva AT, Zackert WE, Zinkel SS, May JM, et al.Cardiolipin fatty acid remodeling regulates mitochondrial function by modifying the electron entry point in the respiratory chain. Mitochondrion 2016;28:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G.Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42(1):332-45. [DOI: 10.1093/ije/dys222] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vincer 1998
- Vincer M, Allen A.Does prophylactic indomethacin inVLBW (<1500 grams birth weight) infants cause cerebral palsy (CP)? Pediatric Research 1998;43:232. [Google Scholar]
Vohr 2003
- Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, et al.School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics 2003;111(4 Pt 1):e340-6. [DOI] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JP.Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012;3(2):111-25. [DOI: 10.1002/jrsm.1045] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 1989
- Wolf WM, Snover DC, Leonard AS.Localized intestinal perforation following intravenous indomethacin in premature infants. Journal of Pediatric Surgery 1989;24(4):409-10. [DOI: 10.1016/s0022-3468(89)80284-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yepes‐Nuñez 2019
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al.Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. [10.1016/j.jclinepi.2019.04.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ystrom 2017
- Ystrom E, Gustavson K, Brandlistuen RE, Knudsen GP, Magnus P, Susser E, et al.Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics 2017;140(5):e20163840. [DOI: 10.1542/peds.2016-3840] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2021
- Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RA, Santesso N, Traversy G, et al.GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. Journal of Clinical Epidemiology 2021;137:163-75. [DOI: 10.1016/j.jclinepi.2021.03.026] [DOI] [PubMed] [Google Scholar]
Zupancic 2006
- Zupancic JA, Richardson DK, O’Brien BJ, Cronin CG, Schmidt B, Roberts R, et al.Retrospective economic evaluation of a controlled trial of indomethacin prophylaxis for patent ductus arteriosus in premature infants. Early Human Development 2006;82:97-103. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mitra 2021
- Mitra S, Gardner CE, MacLellan A, Disher T, Styranko DM, Kuhle SJ, et al.Prophylactic cyclo‐oxygenase inhibitor drugs for the prevention of morbidity and mortality in preterm infants: a network meta‐analysis. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013846. [DOI: 10.1002/14651858.CD013846] [DOI] [PMC free article] [PubMed] [Google Scholar]