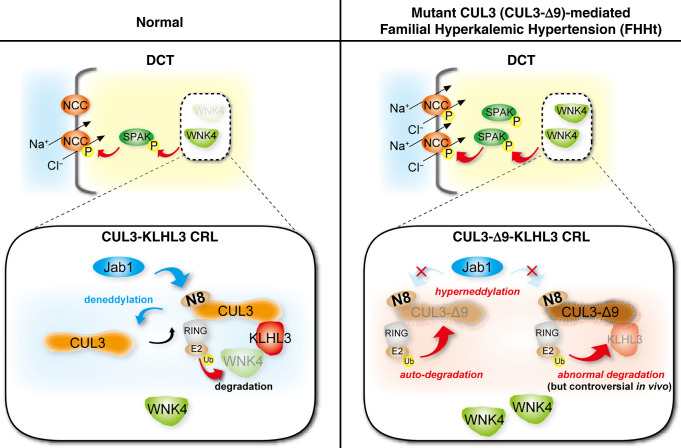
Figure 1.
NCC activity regulation by the CUL3-WNK-SPAK pathway. (Left) In normal conditions the NCC is activated by SPAK, which is in turn activated by WNK4. WNK4 abundance is determined by activity of a CRL consisting of the scaffold Cullin 3 (CUL3), the substrate adaptor Kelch-like 3 (KLHL3), and a RING ubiquitin ligase. The ubiquitin-like protein NEDD8 (N8) is added by to CUL3 by NEDD8 activating enzyme (NAE, not shown), and removal is catalyzed by c-Jun activation domain-binding protein-1 (JAB1). Cycling of NEDD8 addition (neddylation) and removal (deneddylation is essential to maintain CRL stability and activity. (Right) In FHHt, WNK4 accumulates, resulting in hyperactivation of NCC. The precise mechanism by which CUL3 mutations cause WNK4 accumulation has remained unclear. Most CUL3 mutations that cause FHHt cause mis-splicing that results in deletion of exon 9 and generation of a form of CUL3 with a 57 amino acid deletion (CUL3-Δ9). CUL3-Δ9 is unable to interact with JAB1, leading to hyperneddylation. In vitro, this leads to CUL3-Δ9 autoubiquitination. Consistent with this, CUL3-Δ9 expression is extremely low in several CUL3-Δ9–expressing mouse models. In vitro, CUL3-Δ9–mediated degradation of KLHL3 has also been reported, but this has not been consistently found. The aim of this study was to resolve this issue and determine the mechanisms underlying CUL3-Δ9–mediated FHHt.