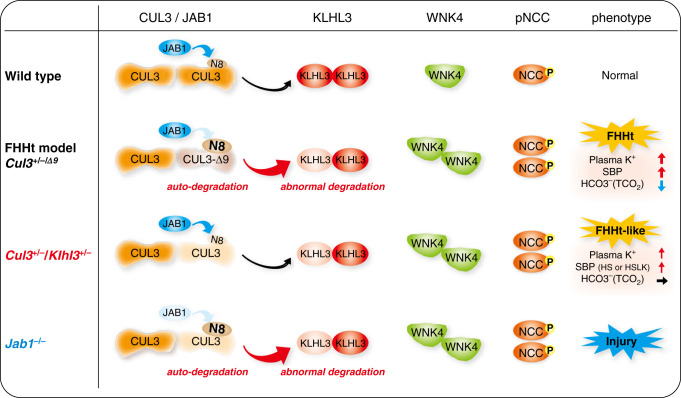
Figure 7.
Renal mechanisms of CUL3-Δ9–mediated FHHt. Wild type, under normal conditions, CUL3 constitutively degrades KLHL3 at a low level, and forms a normal Cullin-Ring ubiqutin ligase with KLHL3 that mediates WNK4 degradation. CUL3 is activated by covalent linkage of the ubiquitin-like protein NEDD8 (N8), which is removed by the enzyme JAB1. Cycling of N8 conjugation (neddylation) and removal (deneddylation) is required for stability of the Cullin-Ring ubiqutin ligase. FHHt models, in CUL3 heterozygous mice expressing CUL3-Δ9 from a transgene (Cul3+/−/Δ9) and knock-in mice with exon 9 deletion (CUL3WT/Δex9), CUL3-Δ9 cannot interact with JAB1, resulting in hyperneddylation. This leads to CUL3-Δ9 autoubiquitination and degradation, and induces KLHL3 degradation. Abundances of WNK4 and pNCC increase, leading to an FHHt phenotype. Cul3+/−/Klhl3+/−, combination of lower CUL3 and KLHL3 abundance results in increased abundance WNK4 and pNCC, and an FHHt-like phenotype. The full FHHt phenotype may require additional effects such as sequestration of adaptors in non-functional complexes and formation of unstable WT-CUL3/CUL3-Δ9 heterodimers. Jab1−/−, acute Jab1 disruption reduces abundances of CUL3 and KLHL3, leading to increased abundances of WNK4 and pNCC. However, due to effects along the whole nephron, there is renal injury rather than an FHHt phenotype.