Abstract
T cells play essential roles in antitumor therapy. Via gene engineering technique to enhance tumor-antigen specificity, patient peripheral blood-derived T cells (PBT) show encouraging clinical outcomes in treating certain blood malignancies. However, the high costs, functionality exhaustion, and disease-condition-dependent availability of PBT prompt the attempts of exploring alternative T cell sources. Theoretically, induced T cells from pluripotent stem cells (PSC) are ideal candidates that integrate plenty of advantages that primary T cells lack, including unlimited off-the-shelf cell source and precision gene editing feasibility. However, researchers are still struggling with developing a straightforward protocol to induce functional and immunocompetent human T cells from PSC. Based on stromal cell-expressing or biomaterial-presenting Notch ligands DLL1 or DLL4, natural and induced blood progenitors can differentiate further toward T lineage commitment. However, none of the reported T induction protocols has yet translated into any clinical application, signaling the existence of numerous technical barriers for regenerating T cells functionally matching their natural PBT counterparts. Alternatively, new approaches have been developed to repopulate induced T lymphopoiesis via in vivo reprogramming or transplanting induced T cell precursors. Here, we review the most recent progress in the T cell regeneration field, and the remaining challenges dragging their clinical applications.
Keywords: Hematopoietic stem/progenitor cells, Notch, Pluripotent stem cells, T cell regeneration
1. LIMITS OF PATIENT PERIPHERAL BLOOD T CELLS FOR IMMUNOTHERAPY
Antigen-specific T cell immunotherapy, such as chimeric antigen receptor (CAR)-T cell therapy, has been broadly used in treating certain tumors, particularly blood malignancies.1 The prominent T cell source for immunotherapy is from individual patients, which is time- and cost-consuming. Besides, there is a reality that a large proportion of tumor patients exhibits compromised T cell functionality,2–5 partially due to heavy tumor burden and chemotherapy.6,7 Meanwhile, primary patient peripheral blood-derived T cells (PBT) always face in vitro manipulating problems, including imprecise and low-efficiency editing and even functionality exhaustion introduced by in vitro culture, which restricts the universal application of PBT in immunotherapy. Thus, it is urgent to explore regenerated cell alternatives to PBT.
2. APPROACHES OF T CELLS REGENERATION
2.1. In vitro induction of T cells from natural hematopoietic stem/progenitor cells (HSPC)
Hematopoietic stem cell-derived progenitors in the bone marrow migrate to thymus and develop into mature T cells in the presence of essential niche factors largely provided by thymic stromal cells and antigen-presenting cells.8–10 Numerous attempts of mimicking the T cell development microenvironment in vitro have been focusing on searching stromal cell alternatives and signaling molecules replacing natural thymus counterparts. The pioneering finding in these attempts is that mouse OP9 stromal cells expressing the Notch ligand DLL1 induced T cell lineage commitment in vitro from natural mouse hematopoietic progenitor cells.11 The same research group further extended their induction protocol to obtain human T cells from natural human blood progenitor cells (CD34+CD38−) in the presence of OP9 stromal cells expressing human NOTCH1 ligand DLL1.12 Regarding the animal origin of OP9 cell line and the MHC-I restriction nature,11,13 the T cell derivatives induced by OP9-DLL1 stromal cells are largely CD8SP T cells carrying host-incompatible TCRs that inevitably encounter immunocompetence problem in vivo. Besides, the OP9-DLL1 induction system has a flaw of positive selection weakness, which leads to the generation of very rare naïve CD8SP T cells.14,15
2.2. Generation of antigen-specific T cells from pluripotent stem cells in vitro
The availability of natural HSPC, such as bone marrow cells or umbilical cord blood cells, is still limited and cannot meet the universal requirement of translational application for precision medicine. The successful reprogramming of somatic cells into induced pluripotent stem cells (iPSC), which possess similar characteristics of embryonic stem cells, theoretically provides an unlimited cell source for generating induced T cells (iT cells).16 The numerous advantages of iPSC, including the avoidance of ethical issues and precise gene-editing feasibility, attract researchers to develop new approaches for producing off-the-shelf T cell products from iPSC.
Zuniga-Pflucker and his collaborators extended their application of OP9-DLL1 induction protocol to pluripotent stem cells (PSC, including ESC/iPSC) and successfully produced induced T cells from these cells in vitro,17 which paves the way for producing gene-edited T cells from iPSC. However, other scientists also questioned the variations of T lineage potential of induced HSPC from human PSC,18,19 highlighting that more factors curbing the reproducibility need to be resolved. Antigen-specific CD8+ T cells from HLA-A24-positive patient with chronic HIV-1 infection were reprogrammed into iPSC, which were able to be differentiated to antigen-specific CD8+ T cells using the OP9-DLL1 induction protocol.20 Theoretically, this approach prospectively forms an amplification loop to produce bulk antigen-specific T cells from the original single natural antigen-specific T cell. However, a recent report documented that the induced CD8αβ T cells from human T-iPSC lose their antigen specificity due to the activation of additional rearrangements of the T cell receptor (TCR) α loci during the CD4/CD8 double-positive stage, which leads to CD8αα innate-like T cells and mispaired TCRs without antigen-specificity (Fig. 1A).21 Further knockout of Rag recombinase gene RAG2 in the T-iPSC successfully prevented this additional endogenous TCR rearrangement (Fig. 1B).21 However, the potential biological risks of disrupting the endogenous Rag loci are largely unknown. Interestingly, myeloid cell-derived TCR-transgenic iPSC differentiated into antigen-specific T cells without activation of the endogenous TCR α loci, though the mechanism is unknown (Fig. 1C).21 Collectively, these observations lay foundation for the regeneration of antigen-specific T cells from pluripotent stem cells in vitro.
FIGURE 1.
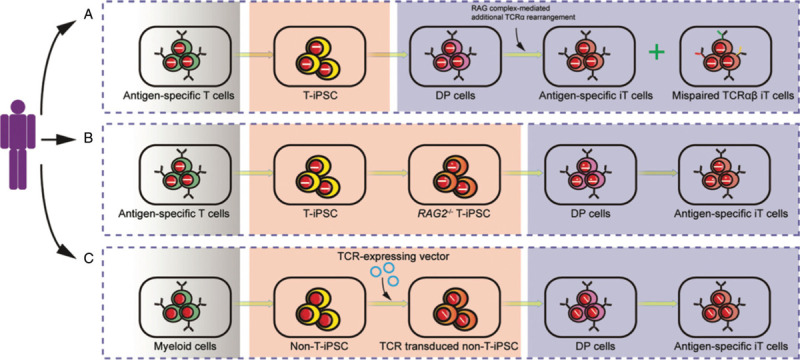
Schema of antigen-specific T cell regeneration from iPSC. Antigen-specific T cells or myeloid cells as the initiating cells are reprogrammed to T-iPSC or non-T-iPSC. (A) Antigen-specific T-cell-derived T-iPSC differentiates into TCRαβ T cells, which partially lose antigen-specificity resulting from mispaired endogenous TCR expression. (B) Knockout of RAG2 at T-iPSC stage eliminates the additional TCR rearrangement and preserves the antigen specificity of induced T cells from T-iPSC. (C) Myeloid-cell-derived non-T-iPSC are transduced with antigen-specific TCR and differentiate into antigen-specific induced T cells with rare activation of endogenous TCR rearrangements.
2.3. Optimization of T cell induction via three-dimensional coculture system
The OP9-DLL1 system, providing a feasible but imperfect method for studying T lineage commitment in vitro, induces a proportion of T cells expressing CD8αα homodimer rather than CD8αβ.22 Fetal thymus organ culture (FTOC) system is a unique and powerful in vitro system that allows intrathymic T-lymphocyte development (Fig. 2A).23,24 This approach can produce homogeneous and naïve TCRβ+ CD8α SP T cells. Thus, the consideration of a 3D system seemingly overcomes the flaws of the 2D-OP9-DLL1/DLL4 induction. However, the expression levels of Ccr7 and Ccr9 in these T cells from the 3D system are lower than their natural naïve T cells, which indicates that these regenerated T cells might have migration problem in vivo. Considering the complexity of 3D FTOC coculture, scientists recently developed an artificial thymic organoid (ATO) system, which includes a stromal cell line named MS5-DLL4 that supports the positive selection of human PSC-derived DP precursors to naïve CD3+TCRαβ+CD8αβ+ T cells in vitro (Fig. 2B).25 There are also discrepancies between the iPSC-ATO-derived T cells and their natural counterparts, as iPSC-ATO-derived T cells showed shorter CDR3 lengths probably due to the absent expression of DNTT (encoding TdT) at DP stage, although the biological consequences of the shorter CDR3 in the regenerated T cells remain unknown. Of note, the current feeder cell lines for T cell induction are from animal source, translationally adding safety concerns for clinical applications.
FIGURE 2.
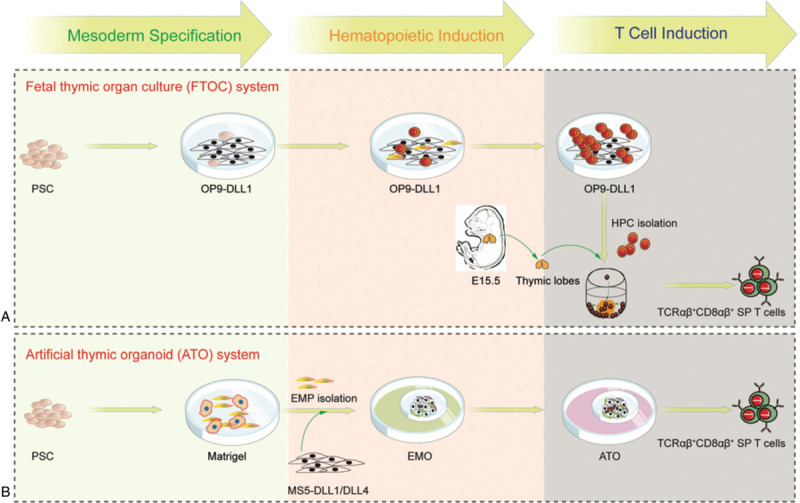
T lineage commitment from PSC-derived cell in 3D coculture systems. (A) In FTOC system, PSC are cocultured with OP9-DLL1 for mesoderm specification, hematopoietic induction, and then T cell progenitor induction. To avoid the regeneration of abnormal T lymphocytes, T cell progenitors are cultured with thymic lobes from E15.5 murine embryos. (B) In ATO system, mesoderm specification is initiated from PSCs on matrigel to produce CD326−CD56+ embryonic mesodermal progenitors (EMPs) that are cocultured with MS5-DLL1 or MS5-DLL4 as an embryonic mesodermal organoid (EMO) and artificial thymic organoid (ATO) system. Hematopoietic induction and T cell induction are induced by medium change at different stages.
2.4. Serum- and feeder-free condition for T cell induction
Serum and feeder cells used for conventional T cell induction not only introduce efficiency variations among different experimental batches or experimental operators but also bring up safety issues such as biocontamination. Scientists have been exploring alternative to these materials in T cell induction protocols. In a new developed ATO system, MS5-hDLL1/DLL4 feeder lines on serum-free culture condition can support the T lineage commitment of HSPC or PSC.25,26 In the absence of serum and feeder cells, the combination of immobilized DLL1, fibronection, and appropriate growth factors induces the expansion of human common lymphoid precursor-like (CD34+CD7+CD45RA+) cells and promotes the generation of thymus-repopulating T cell precursors.27 In addition, Fc-DLL4 fusion protein can expand mouse early T cell precursors (ETPs) but arrest the late T cell development.28 In a recent study, subcutaneous injection of the macroporous hydrogel, a biomaterial-based bone marrow-like scaffold releasing BMP2 and presenting DLL4, at the time of hematopoietic stem cell transplantation, facilitates the seeding of T cell precursors in thymus and T cell regeneration in vivo.29 This system provides a perspective paradigm for improving T cell regeneration in vivo, which is completely serum- and feeder-free.
2.5. Regeneration of immunocompetent T cells in vivo
The introduction of antigen-specific TCR and silencing of endogenous TCR rearrangements largely rescue the translational potential of in vitro induced T cells that lacks host-immunocompetent TCR repertoire. To date, there are no comparative studies yet to conclude that whether the activity of regenerated T cells in vitro is comparable to the PBT. Considering the administration of multiple doses of antigen-specific T cells in a short time window in treating the tumor-bearing mice, the activity exhaustion of regenerated T cells is still a big barrier.21 In addition to their application in antitumor therapy, functional T cells are also needed as T lymphopoiesis surrogate in certain patients, such as immune-deficient or immune-impaired patients. Natural HSPC-derived T cell precursors possess thymus-homing capacity (Fig. 3A). Unfortunately, the PSC-derived T cell precursors showed a defect of thymus-seeding ability in vivo (Fig. 3B).17 Thus, it is a central battleground for scientists to regenerate genuine T cell precursors in vitro that can seed the thymus and output naïve T cells in vivo. Alternatively, we have recently revealed that mouse pro-pre-B cells can be converted to ETPs in vivo by enforcing Hoxb5 expression. These induced ETPs successfully seeded the thymus and developed into mature T cells (Fig. 3C).30 More recently, we established a straightforward method of preferentially achieving T lymphopoiesis in vivo from mouse PSC source (Fig. 3D).31 In this study, we performed functional screening of genuine T cell precursors and identified that the synergistic expression of Runx1 and Hoxa9 at the short time window of endothelial-to-hematopoietic transition and emergence of hematopoietic progenitors in vitro leads to the generation of robust T cell precursors, which possess the thymus-seeding ability. The promoting role of Runx1 and Hoxa9 in T lineage commitment is striking as a single hemogenic endothelial cell-derived blood progenitor colony can efficiently reconstitute T lymphopoiesis in vivo. Taken together, Runx1 and Hoxa9 promote the generation of genuine T cell precursors induced by OP9-DLL1 system as the essential intrinsic drivers. Regeneration of T lymphopoiesis in vivo from PSC source is promising for correcting or rescuing the inherited- or disease-weakened/impaired T cell immune system in patients.
FIGURE 3.
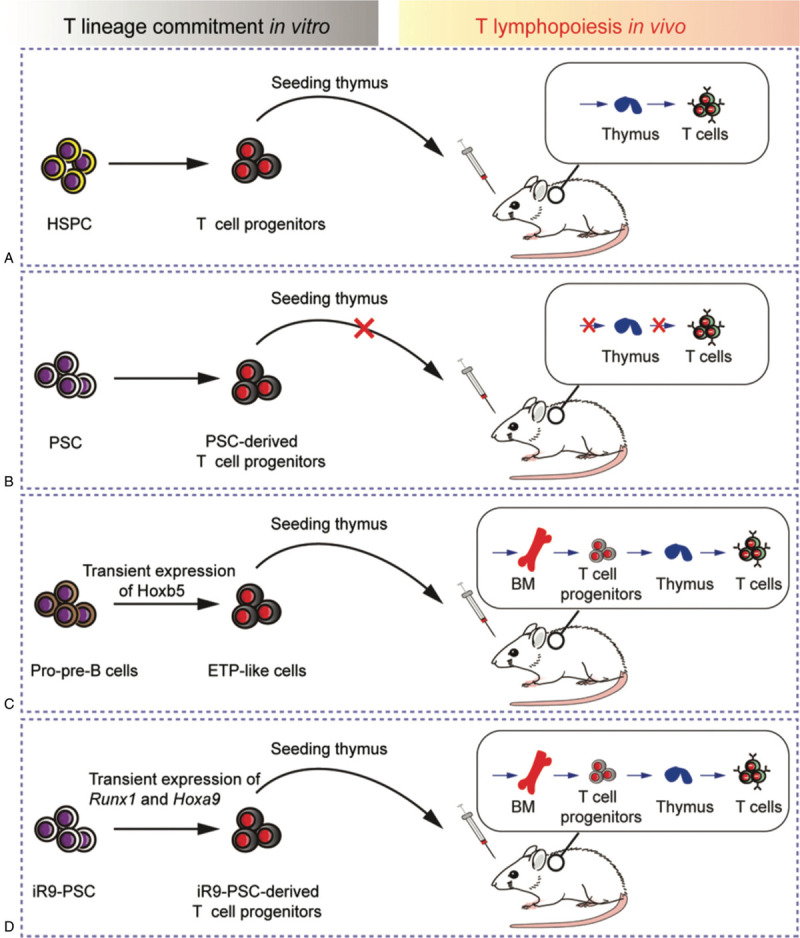
The T-lymphopoiesis potential of T cell progenitors from different approaches. (A) After T lineage induction with OP9-DLL1/DLL4 cell monolayer or with MS5-DLL1/DLL4 for 3D ATO culture in vitro, natural HSPC-derived T cell progenitors can migrate into thymus and develop into T cells in host. (B) PSC-derived T cell progenitors lack the thymus-seeding ability and fail to reconstitute T lymphopoiesis in vivo. (C) Pro-pre-B cells with enforced expression of Hoxb5 can be converted to early T cell precursors (ETPs), which seed the thymus and develop into mature T cells. (D) Synergistic and transient expression of Runx1 and Hoxa9 during the hematopoietic induction process from PSC grants the thymus-seeding capacity for PSC-derived T cell progenitors, which repopulate T regular in vivo. iR9: inducible expression of Runx1 and Hoxa9.
3. CONCLUSION
Scientists have achieved great progress in generating phenotypic CD8SP T cells from either natural hematopoietic progenitors or pluripotent stem cells, largely based on mouse feeder cell lines expressing Notch ligands. However, it remains challenging to produce sufficient doses of functional and immunocompetent human CD8SP T cells in vitro that satisfy clinical requirements. Besides antitumor therapy, there is another large and general health demand that, in disease-caused compromised T immune system (e.g., HIV infection), regeneration of functional T lymphopoiesis in vivo is urgently needed. Thus, obtaining engraftable T cell precursors from PSC can further extend the application of T cell therapy to a broader range of immune-related diseases, including inherited and disease-caused immunodeficiencies and abnormalities.
ACKNOWLEDGMENTS
This work is supported by the Key R&D program from Ministry of Science and Technology of China (2019YFA0110203), Major Research and Development Project of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110104006), the Health and Medical Care Collaborative Innovation Program of Guangzhou Scientific and Technology (201803040017), the Science and Technology Planning Project of Guangdong Province (2017B030314056), and the National Natural Science Foundation of China (31471117, 81470281, 31600948).
REFERENCES
- [1].Gauthier J, Yakoub-Agha I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: clinical data to date, current limitations and perspectives. Curr Res Transl Med 2017;65 (3):93–102. [DOI] [PubMed] [Google Scholar]
- [2].Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 2003;98 (5):1089–1099. [DOI] [PubMed] [Google Scholar]
- [3].Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169 (5):2756–2761. [DOI] [PubMed] [Google Scholar]
- [4].Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005;65 (6):2457–2464. [DOI] [PubMed] [Google Scholar]
- [5].Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003;9 (2):606–612. [PubMed] [Google Scholar]
- [6].Das RK, Vernau L, Grupp SA, Barrett DM. Naive T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov 2019;9 (4):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singh N, Perazzelli J, Grupp SA, Barrett DM. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med 2016;8 (320):320ra323. [DOI] [PubMed] [Google Scholar]
- [8].Ciofani M, Zuniga-Pflucker JC. The thymus as an inductive site for T lymphopoiesis. Ann Rev Cell Dev Biol 2007;23:463–493. [DOI] [PubMed] [Google Scholar]
- [9].Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol 2003;21:139–176. [DOI] [PubMed] [Google Scholar]
- [10].Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol 2007;25:649–679. [DOI] [PubMed] [Google Scholar]
- [11].Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 2002;17 (6):749–756. [DOI] [PubMed] [Google Scholar]
- [12].La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood 2005;105 (4):1431–1439. [DOI] [PubMed] [Google Scholar]
- [13].Awong G, Herer E, La Motte-Mohs RN, Zuniga-Pflucker JC. Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature. BMC Immunol 2011;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis 2004;33 (3):227–232. [DOI] [PubMed] [Google Scholar]
- [15].de Pooter R, Zuniga-Pflucker JC. T-cell potential and development in vitro: the OP9-DL1 approach. Curr Opin Immunol 2007;19 (2):163–168. [DOI] [PubMed] [Google Scholar]
- [16].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126 (4):663–676. [DOI] [PubMed] [Google Scholar]
- [17].Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol 2004;5 (4):410–417. [DOI] [PubMed] [Google Scholar]
- [18].Martin CH, Woll PS, Ni Z, Zuniga-Pflucker JC, Kaufman DS. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood 2008;112 (7):2730–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li X, Xia C, Wang T, et al. Pyrimidoindole derivative UM171 enhances derivation of hematopoietic progenitor cells from human pluripotent stem cells. Stem Cell Res 2017;21:32–39. [DOI] [PubMed] [Google Scholar]
- [20].Nishimura T, Kaneko S, Kawana-Tachikawa A, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell 2013;12 (1):114–126. [DOI] [PubMed] [Google Scholar]
- [21].Minagawa A, Yoshikawa T, Yasukawa M, et al. Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell Stem Cell 2018;23 (6):850–858. e854. [DOI] [PubMed] [Google Scholar]
- [22].McNicol AM, Bendle G, Holler A, et al. CD8alpha/alpha homodimers fail to function as co-receptor for a CD8-dependent TCR. Eur J Immunol 2007;37 (6):1634–1641. [DOI] [PubMed] [Google Scholar]
- [23].Nitta T, Ohigashi I, Takahama Y. The development of T lymphocytes in fetal thymus organ culture. Methods Mol Biol 2013;946:85–102. [DOI] [PubMed] [Google Scholar]
- [24].Vizcardo R, Klemen ND, Islam SMR, et al. Generation of tumor antigen-specific iPSC-derived thymic emigrants using a 3D thymic culture system. Cell Rep 2018;22 (12):3175–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Montel-Hagen A, Seet CS, Li S, et al. Organoid-induced differentiation of conventional T cells from human pluripotent stem cells. Cell Stem Cell 2019;24 (3):376–389. e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seet CS, He C, Bethune MT, et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat Methods 2017;14 (5):521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(−) cord blood cells. J Clin Invest 2002;110 (8):1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ikawa T, Hirose S, Masuda K, et al. An essential developmental checkpoint for production of the T cell lineage. Science 2010;329 (5987):93–96. [DOI] [PubMed] [Google Scholar]
- [29].Shah NJ, Mao AS, Shih TY, et al. An injectable bone marrow-like scaffold enhances T cell immunity after hematopoietic stem cell transplantation. Nat Biotechnol 2019;37 (3):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang M, Dong Y, Hu F, et al. Transcription factor Hoxb5 reprograms B cells into functional T lymphocytes. Nat Immunol 2018;19 (3):279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guo R, Hu F, Weng Q, et al. T lymphopoiesis from pluripotent stem cells by defined transcription factors at single cell resolution. bioRxiv 2019;660977. [Google Scholar]


