Figure 4.
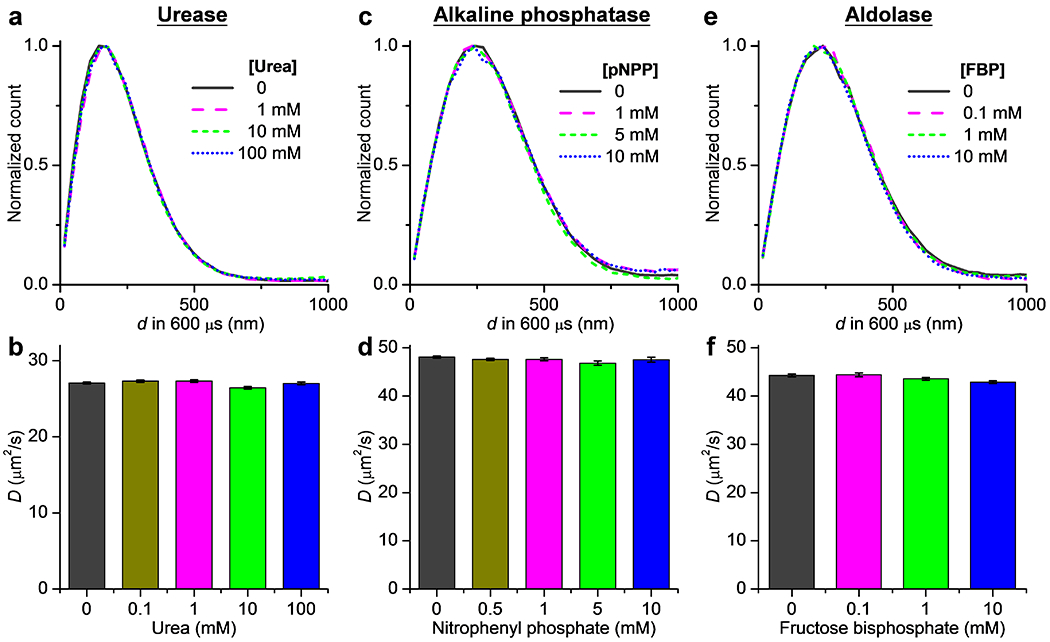
Results on urease, alkaline phosphatase, and aldolase also show no changes in single-molecule displacements under catalytic turnover. (a) Normalized distributions of the measured 600-µs single-molecule displacements for Cy3B-labeled urease in PBS, with and without the addition of urea at different concentrations. (b) MLE-determined D values at different urea concentrations. Error bars: 95% confidence intervals of MLE. (c,d) Similar to that in (a,b), but for Cy3B-labeled alkaline phosphatase in 0.1 M Tris-HCl (pH = 9.8) with the addition of 1 mM MgCl2 and 20 µM ZnCl2, with p-nitrophenyl phosphate (pNPP) as the substrate. (e,f) Similar to that in (a,b), but for Cy3B-labeled aldolase in 0.1 M HEPES (pH = 7.4), with fructose 1,6-bisphosphate (FBP) as the substrate.
