Abstract
Background:
Bovine lactoferrin addition to regimens of Helicobacter pylori treatment has been tried, with conflicting results.
Aim:
To assess the effect of bovine lactoferrin in addition to the anti-H. pylori treatment.
Methods:
We enrolled 400 H. pylori-infected patients who were randomized into 4 equal groups: (A): proton-pump-based triple therapy (PpTT) for 2 weeks, (B): sequential therapy for 2 weeks, (C): proton-pump-based triple therapy plus bovine lactoferrin for 2 weeks, and (D): sequential therapy plus bovine lactoferrin for 2 weeks.
Results:
In the per-protocol analysis, the success in groups A, B, C, and D were 70.3%, 82.8%, 85.6%, and 94.5%, respectively (P < .001). The treatment success rate for the sequential therapy plus bovine lactoferrin regimen was significantly higher than that with sequential therapy alone (94.5% vs. 82.8%, P = .013). The same applied for proton-pump-based triple therapy (85.6% vs. 70.3%, P = .014). The addition of bovine lactoferrin and the presence of endoscopic corpus gastritis were independent predictors for successful eradication of H. pylori.
Conclusion:
Bovine lactoferrin could hasten the effectiveness of the proton-pump-based triple therapy or sequential therapy for H. pylori eradication.
Keywords: Antibiotic resistance, eradication therapy, H. pylori, lactoferrin.
Main Points
Bovine lactoferrin (bLf) could significantly improve the efficacy of the proton-pump-based triple therapy (PpTT) or sequential therapy (ST) for Helicobacter pylori eradication.
The addition of bLf to H. Pylori regimens is safe, with no adverse events or drug-related discontinuation.
Introduction and Aim
Helicobacter pylori (H. pylori) is a Gram-negative curved flagellar microorganism that populates the stomach.1 Helicobacter pylori infection is linked to many upper gastrointestinal diseases, including chronic gastritis, peptic ulceration, gastric carcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma.2
The elimination of H. pylori is not only an important component in the healing of peptic ulcers, it also decreases their recurrence, reduces the rate of gastric carcinoma recurrence after the early gastric cancer resection, and mediates the regression of MALT lymphoma.3
Eradication of H. pylori is currently recommended in numerous guidelines for the treatment of all these associated diseases.4,5 Proton-pump-based triple therapy (PpTT) is the most commonly accepted H. pylori treatment, which consists of a twice-daily proton pump inhibitor (PPI), clarithromycin, and amoxicillin/metronidazole for 7 days.6
The reported efficacy of PpTT in the extermination of H. pylori is 74-76%, but it has dropped considerably. In a recent study, the rate of H. pylori eradication by PpTT significantly decreased from ~87% prior to 2007 to ~80% between 2008 and 2010.7 Therefore, different strategies to resolve the treatment failure of PpTT have been suggested. These protocols include the bismuth-based quadruple therapy, extension of therapy duration, the use of a sequential therapy (ST) composed of 4 drugs [PPI and amoxicillin for the first 5 days augmented by a PPI plus clarithromycin and metronidazole (or tinidazole) regimen for the next 5 days], concurrent therapy, hybrid therapy, and the use of new antibiotics (e.g., levofloxacin).8
In the last 2 decades, there has been rising interest in the possible role of bovine lactoferrin (bLf) in the treatment and detection of different gastrointestinal diseases. As a significant factor in the host’s defense against a wide variety of bacteria, bLf is a multipurpose iron-binding glycoprotein that is found in the milk, body fluids (e.g., saliva, tears, bile), pancreatic and seminal fluids, and the granules of the polymorphonuclear leukocytes in humans and bovines.9 A few studies have reported the role of bLf as an add-on therapy for eradication of H. pylori, with conflicting results.10,11 Therefore, we designed this randomized controlled trial to assess the role of bLf as an add-on in the treatment regimens to eradicate H. pylori infection, either PPTT or ST.
Materials and Methods
In a parallel, randomized, controlled, superiority study design, we calculated the sample size at a statistical power of 0.95, a two-tailed α level of 0.05, and a 20% effect of size. Assuming an eradication rate of 75% among the non-lactoferrin group based on previous studies,7 the calculated size (n = 354) was corrected for an assumed 5% drop-out (multiplied by 1.11 according to Wittes J.).12 Therefore, we targeted the inclusion of 400 patients.
Patients with symptoms related to the upper gastrointestinal tract (GIT) and a positive test for H. pylori infection, who presented to the gastroenterology and general internal medicine clinics at our institute were evaluated for eligibility (n = 723). The exclusion criteria were the intake of PPI, H2-blockers, intake of bismuth-based formulae 2 weeks preceding and/or antibiotics a month preceding the enrollment, active GIT bleeding at the time of H. pylori testing, prior eradication treatment, and history of gastrectomy. Patients with equivocal H. pylori stool antigen (HpSAg) results, patients with advanced liver (Child-Pugh class B or C) or renal diseases (glomerular filtration rate <60 mL/min/1.73 m2) and any form of malignancy, proven clarithromycin or penicillin allergy, and pregnant/lactating females were also excluded.
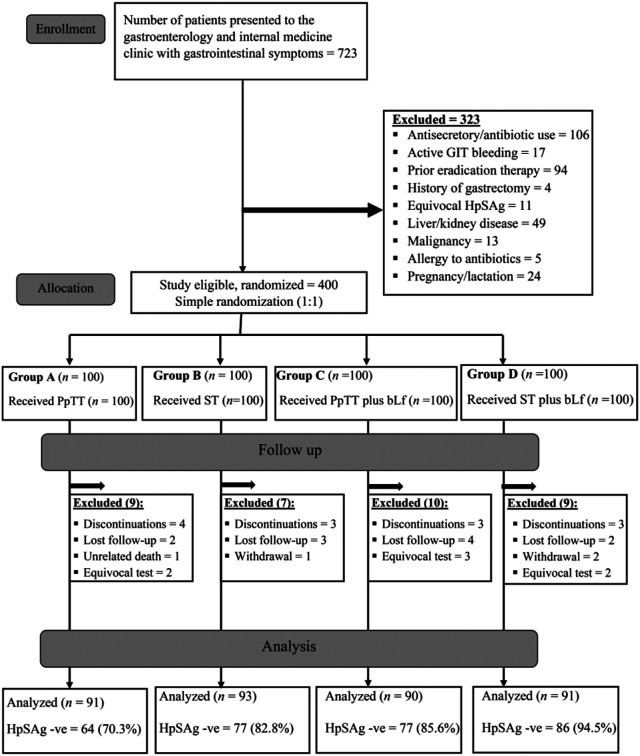
The study was conducted in agreement with the provisions of the Declaration of Helsinki, as revised in 2013, and the Good Clinical Practice Guidelines. Informed consent was obtained from all participants. The study obtained the institutional ethical committee approval (IRB: 0304432), and was also registered on the clinicaltrial.gov registry with the number: (NCT04445948) (https://register.clinicaltrials.gov/prs/app/template/Preview.vm?epmode=Edit&popup=true&uid=U0004MW7&ts=3&sid=S0009ZUI&cx=-zf8vdt). Figure 1 shows the CONSORT flow diagram of the study.
Figure 1.
The CONSORT flowchart of the study. GIT, gastrointestinal tract; HpSAg, Helicobacter pylori stool antigen; PpTT, proton-pump-based triple therapy; ST, sequential therapy.
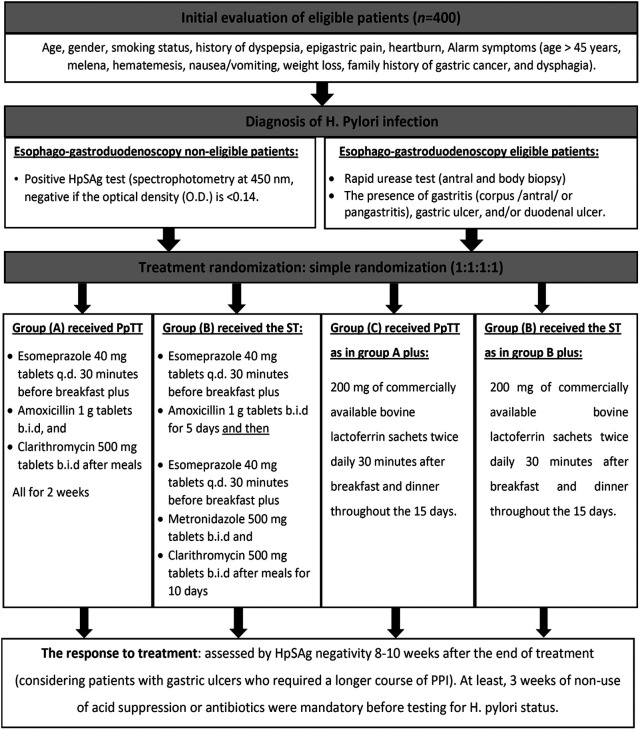
The stepwise methodology including patient evaluation, H. pylori diagnosis, treatment allocation, and response evaluation are shown in Figure 2. The patients were expected to have received at least 90% of the treatment course to be defined as treatment-compliant. Patients were encouraged to report any adverse events during the treatment course by directly contacting the study team. To ensure compliance to treatment, the patients were asked to check against each pill/sachet dose in a pre-prepared calendar with a daily checklist, and were asked to bring the empty medication packages back to us as assurance of regular intake.
Figure 2.
A graphical presentation of the methodological approach of the study. HpSAg, Helicobacter pylori stool antigen; OD, optical density; PpTT, proton-pump-based triple therapy; PPI, proton pump inhibitors; ST, sequential therapy.
The primary outcome of the current work was to evaluate the safety as well as the benefit of adding bLf to both PpTT as well as ST in improving the eradication rates of H. pylori infection.
Statistical Analysis
The sample size was calculated using the G-Power software (v. 3.1.9.4, Universität Düsseldorf, Germany). Statistical analysis was done using the IBM SPSS software package version 26.0 (Armonk, NY: IBM Corp). Our analysis was a per-protocol (PP) analysis. Qualitative data were described using a number (%) while quantitative data were described using range (minimum and maximum), mean, and standard deviation, as appropriate. The normality of data distribution was assessed by the Kolmogorov–Smirnov test. A chi-square test was used for categorical analysis, for comparison between the different groups. The ANOVA test was used to compare between means ± SD of the 4 groups. Univariate and multivariate analyses were done to explore predictors of response to treatment. Statistical significance was assessed at P < .05. All calculated P values were two-tailed.
Results
The study allocation began with 400 patients allocated randomly to 4 equal groups, with 100 patients in each group. During the study period, 35 (8.75%) patients dropped out (13 patients discontinued the treatment, 11 patients were lost to follow-up, 3 patients withdrew from the study, 7 patients had equivocal HpSAg test results, and 1 patient died in a road traffic accident) (Figure 1).
Per-Protocol Analysis
The study analysis per-protocol (PP) included 365 patients [group A (n = 91), B (n = 93), C (n = 90), and D (n = 91)]. The demographic and baseline clinical data are shown in Table 1.
Table 1.
Comparison Between the 4 Groups Studied, According to Different Baseline Parameters
| Parameters |
Group A (n = 91) |
Group B (n = 93) |
Group C (n = 90) |
Group D (n = 91) |
Test of Sig. |
P * |
|---|---|---|---|---|---|---|
| Age (Mean ± SD) | 42.22 ± 10.47 | 42.28 ± 10.59 | 42.71 ± 9.69 | 42.84± 10.1 | F = 0.08 | .97 |
| Male, n (%) | 54 (59.3%) | 50 (53.8%) | 49 (54.4%) | 40 (44%) | χ2 = 4.55 | .21 |
| Smokers, n (%) | 37 (40.7%) | 46 (49.5%) | 44 (48.9%) | 46 (50.5%) | χ2 = 2.26 | .52 |
| Clinical symptoms, n (%) | ||||||
| Dyspepsia | 32 (35.2%) | 37 (39.8%) | 37 (41.1%) | 36 (39.6%) | χ2 = 0.77 | .86 |
| Epigastric pain | 27 (29.7%) | 25 (26.9%) | 24 (26.7%) | 24 (26.3%) | χ2 = 0.32 | .96 |
| Heartburn | 8 (8.8%) | 9 (9.7%) | 3 (3.3%) | 9 (9.9%) | χ2 = 3.6 | .31 |
| Melena | 2 (2.2%) | 5 (5.4%) | 8 (8.9%) | 5 (5.4%) | χ2 = 3.9 | .27 |
| Hematemesis | 4 (4.3%) | 5 (5.4%) | 8 (8.9%) | 4 (4.4%) | χ2 = 2.7 | .52 |
| Nausea and vomiting | 14 (15.4%) | 10 (10.8%) | 5 (5.6%) | 8 (8.8%) | χ2 = 5.0 | .17 |
| Weight loss | 2 (2.2%) | 1 (1.1%) | 2 (2.2%) | 3 (3.2%) | χ2 = 1.1 | .79 |
| Dysphagia | 2 (2.2%) | 3 (3.3%) | 2 (2.2%) | 1 (1.1%) | χ2 = 97 | .81 |
| Endoscopic findings | n = 47 | n = 53 | n = 45 | n = 42 | ||
| Duodenal ulcer | 4 (8.5%) | 8 (15.1%) | 7 (15.6%) | 8 (19%) | χ2 = 2.12 | .55 |
| Gastric ulcer | 6 (12.8%) | 3 (5.7%) | 1 (2.2%) | 2 (4.8%) | χ2 = 4.7 | .19 |
| Antral gastritis | 17 (36.2%) | 17 (32.1%) | 15 (33.3%) | 14 (33.3%) | χ2 = 0.17 | .98 |
| Corpus gastritis | 20 (42.6%) | 22 (41.5%) | 19 (42.2%) | 19 (45.2%) | χ2 = 0.15 | .99 |
| Pan gastritis | 5 (10.6%) | 10 (18.9%) | 11 (24.4%) | 9 (21.4%) | χ2 = 3.2 | .36 |
| Diagnosis of Helicobacter pylori | ||||||
| RUT/HpSAg | 47/44 | 53/40 | 45/45 | 42/49 | χ2 = 2.23 | .53 |
*Statistically significant at P ≤ .05. HpSAg, H. pylori stool antigen; Min, minimum; Max, Maximum; n, number; RUT, rapid urease test; SD, standard deviation; χ 2, chi-square test; F, F value for ANOVA test.
Our groups were matched, as there was no difference in terms of age, gender, and clinical data (P > .05).
The most prevalent GIT symptom among all patients was dyspepsia (38.9%), followed by epigastric pain (27.4%). History of GIT bleeding (melena and/or hematemesis) was present in 11.2% of the cases. Endoscopy (and hence, RUT) was done for 187 (51.2%) patients.
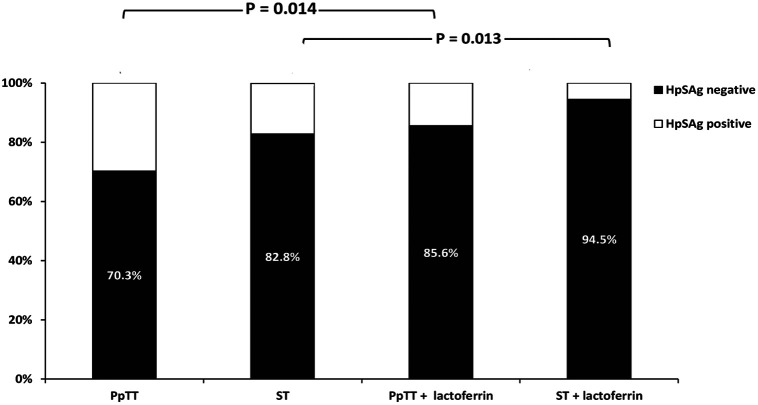
In the follow-up evaluation of treatment response, 304 out of 365 (83.3%) patients were negative for the HpSAg test. In subgroup analysis, the cure rates in groups A, B, C, and D were 70.3%, 82.8%, 85.6%, and 94.5% respectively (P < .001).
The eradication rate in the PpTT plus lactoferrin group was significantly more than the eradication rate among patients who received PpTT alone (85.6% vs. 70.3%, P = .014). The cure rate among patients who received ST plus lactoferrin was significantly higher compared to the rate among those receiving ST alone (94.5% vs. 82.8%, P = .013) (Figure 3). It is worth mentioning that we found the PpTT plus lactoferrin regimen as effective as the ST alone (85.6% vs. 82.8%, P = .61).
Figure 3.
Comparisons between different study groups in terms of the eradication rates, with their statistical significance. HpSAg, Helicobacter pylori stool antigen test; PpTT, proton-pump-based triple therapy; ST, sequential therapy.
Intention-to-Treat Analysis: (n = 400, 100 Patients for Each Group)
In the intention-to-treat analysis (ITT), we considered all the allocated patients, and the dropouts were considered as cases that failed to respond to treatment. The cure rates in groups A, B, C, and D were 64%, 77%, 77%, and 86% respectively (χ 2 = 13.49, P = .004).
The eradication rate among patients who received PpTT plus lactoferrin was significantly high in comparison to the eradication rate among patients who were treated with PpTT alone (77% vs. 64%, P = .04).
The eradication rate among patients who received ST plus lactoferrin was higher than that in patients who received ST alone (86% vs. 77%). However, this was statistically not significant (P = .10).
Regression Analysis
In the univariate and multivariate analyses, the presence of corpus gastritis on endoscopy, as well as the addition of lactoferrin to H. pylori PpTT or ST were independent predictors of H. pylori eradication (P < .001, OR = 12.13, 95% CI: 3.22-45.68; and P = .001, OR = 2.75, 95% CI: 1.51-5.0 respectively) (Table 2).
Table 2.
Univariate and Multivariate Analyses for Predictors of Response to Helicobacter pylori Therapy
| Parameters |
Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| P | 95% CI | P | OR | 95% CI | |
| Gender | .44 | 0.71-2.16 | .11 | 0.86-4.81 | |
| Age | .86 | 0.97-1.02 | .22 | 0.98-1.07 | |
| Smoking | .21 | 0.82-2.52 | .57 | 0.32-1.88 | |
| Gastric ulcer | .12 | 0.10-1.29 | .18 | 0.12-1.49 | |
| Antral gastritis | .29 | 0.30-1.42 | .71 | 0.48-2.84 | |
| Corpus gastritis | .009 | 1.38-10.24 | <.001 | 12.13 | 3.22-45.68 |
| Duodenal ulcer | .18 | 0.62-12.35 | .89 | - | 0.06-1.76 |
| Lactoferrin | <.001 | 1.41-4.86 | .001 | 2.75 | 1.51-5.0 |
Safety and Adverse Reactions
Most of the side effects that led to treatment discontinuation were observed in 13 patients (3.3%, n = 400). These included dizziness (30.8%), headache (23.1%), fatigue (30.8%), nausea (61.5%), taste disturbance (53.8%), and colonic distension (38.5%) (n = 13). We observed no severe or life-threatening adverse reactions or allergic manifestations.
Discussion
Optimization of the H. pylori therapy remains a challenge, and different clinical studies have tried to provide the optimum lines of treatment to achieve better eradication rates. Based on the previously mentioned characteristics of bLf and its efficacy against different microorganisms, and with the conflicting results of previous studies, we designed the current open-label, parallel, randomized clinical study which showed a significant rise in the eradication rates of H. pylori by adding bLf to either the PpTT or the ST regimens.
In the current study, the PP analysis showed that the addition of bLf to the PpTT or ST regimens increased the eradication rate from 70.3% and 82.8% to 85.6% and 94.5%, respectively. Besides, bLf significantly increased the eradication rate of PpTT to be as effective as ST, emphasizing the beneficial effects of adding bLf to H. pylori eradication therapy. Moreover, the ITT analysis showed a significant rise in the eradication rates, from 64% and 77% up to 77%, and 86%, respectively. Although this was statistically insignificant for the ST regimen, it seems clinically significant.
The role of bLf alone in H. pylori management among humans was documented for the first time in 2003 by Di Mario and colleagues. They demonstrated in a recent multicenter study that bLf is an essential adjuvant to a one-week triple-combination therapy for H. pylori extermination.13 Moreover, recombinant lactoferrin has been found to enhance the efficacy of triple-therapy regimens in H. pylori-infected mice. This is following our results. However, our study included a larger number of patients and compared more than a single regimen.14 Similarly, in vitro and in vivo studies have demonstrated an enhanced anti-H. pylori role of Lactobacillus acidophilus.15 Bovine lactoferrin could increase the response to levofloxacin-based therapy to 96%.16
In a meta-analysis by Zou et al.,17 bLf was also proved to enhance the treatment of H. pylori in the ITT analysis of data of 1343 patients in 9 randomized trials. Patients who used lactoferrin were also found to experience medication-related side effects less frequently.
On the contrary, Zullo and colleagues18 reported similarity between PpTT and PpTT plus bLf in recent research from 3 centers. They concluded that there was no increase in the efficacy when amoxicillin was added to bLf. The same effect was noted with ST plus bLf compared to ST alone. It is noteworthy that the only available data about amoxicillin–bLf synergism are based on animal studies.19
The possible mechanisms by which bLf enhances the eradication of H. pylori have been addressed in previous studies. In addition to its immune-modulatory effect, it is well known that bLf has both in vivo and in vitro antimicrobial actions (both bactericidal and bacteriostatic) against Helicobacter species. Due to its iron-binding affinity, bLf also deprives the bacteria of iron utilization, with subsequent antibacterial effect. Moreover, bLf may also have the ability to make structural changes in the microbial cell membrane structure, alter the enzymatic activity, and increase the by-products generated by aerobic metabolism, which also affect the microbial viability. In addition, bLf exerts an effect against the attachment and colonization of H. pylori bacteria in the gastric mucosa, with a subsequent reduction in organism number as well as the associated inflammatory process.13,20,21
In our study, the ST was more effective than the PpTT, a finding that matches previously reported data. The eradication rate for PpTT was less than the accepted threshold, while ST could be an effective and appropriate option for first-line H. pylori abolition therapy.22 However, Eisig et al.23 in a double-blind, randomized controlled trial concluded that PpTT is as effective as ST.
In our study, we found that the addition of bLf to PpTT resulted in an eradication rate similar to ST. Antibiotic resistance has been an emerging problem against H. pylori treatment. In a systematic review and meta-analysis by Savoldi et al.,24 the pooled prevalence of primary clarithromycin and metronidazole resistance in our community approaches ~56% and 63%, respectively. The quadruple regimen “bismuth-based” is a better alternative when clarithromycin and metronidazole resistance rates exist, but unfortunately, bismuth is not available in our country. This explains the low eradication rates for PpTT, which could be overcome by the addition of bLf in our study. Based on this result, we postulate that PpTT plus bLf would be an effective and cheap initial choice instead of the initiation of ST to decrease the emergence of antibiotic resistance, and that ST could be kept as a rescue solution for difficult cases, especially with the low cost of bLf ( ~15$ for a 14-day course).
In the current study, we used esomeprazole 40 mg as a once-daily dose in the 4 groups. Esomeprazole, the S-isomer of omeprazole, provides a better acid suppression effect than omeprazole and pantoprazole, with proven higher activity against H. pylori.25,26 A single dose of 40 mg q.d. of esomeprazole has been found as effective as a 20 mg b.i.d. dose, with the privilege of better compliance for a single daily dose. These data agree with our methodology and results.27,28
In the current study, univariate and multivariate analyses proved that the addition of lactoferrin to the treatment regimens and the presence of corpus-antral gastritis by endoscopy were the only independent predictors of H. pylori cure. In a previous study by Georgopoulos et al., they studied the factors which may influence H. pylori treatment outcome. They concluded, in accordance with our results, that the presence of corpus gastritis and the absence of lymphoid hyperplasia in the gastric wall were positive predictors for eradication.29 It has been claimed that the presence of body gastritis will decrease gastric acid production with subsequent elevation of the pH in the stomach, favoring the bactericidal effect of antibiotics.24 However, in our study, we did not evaluate the histopathology of the gastric mucosa, a point we consider as a limitation. In conclusion, this RCT shows that the addition of bLf could significantly enhance the efficacy of the PpTT or ST for H. pylori eradication. The PpTT plus bLf therapy could be a valid alternative regimen to the standard H. pylori protocol of treatment.
Funding Statement
The researchers did not receive any funding for this study.
Footnotes
Ethics Committee Approval: The study was approved by the Ethical Committee in the Faculty of Medicine, University of Alexandria, Egypt (IRB: 0304432), and was registered on clinicaltrial.gov registry with an ID: NCT04445948.
Informed Consent: Informed written consent was obtained from all enrolled patients prior to their participation.
Peer Review: Externally peer-reviewed.
Author Contributions: Concept – FH, EA; Design – SL, FH; Supervision – FH, EA, SL; Resources – EA, SL; Materials – FH, SL, EA; Data Collection and/or Processing – FH, SL, EA; Analysis and/or Interpretation – SL, EA; Literature Search – FH, SL, EA; Manuscript Writing – SL, EA; Critical Review – FH, SL, EA.
Conflict of Interest: The authors have declared that no conflicts of interest exist.
References
- 1. . Malfertheiner P, Mégraud F, O’Morain C.European Helicobacter Pylori Study Group (EHPSG), et al. Current concepts in the management of Helicobacter pylori infection — the Maastricht 2-2000 Consensus report. Aliment Pharmacol Ther. 2002;16(2):167–180.. 10.1046/j.1365-2036.2002.01169.x) [DOI] [PubMed] [Google Scholar]
- 2. . Moss SF, Malfertheiner P. Helicobacter, and gastric malignancies. Helicobacter. 2007;12(S1):23–30.. 10.1111/j.1523-5378.2007.00539.x) [DOI] [PubMed] [Google Scholar]
- 3. . Leodolter A, Kulig M, Brasch H.et al. Meta-analysis comparing eradication, healing, and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15(12):1949–1958.. 10.1046/j.1365-2036.2001.01109.x) [DOI] [PubMed] [Google Scholar]
- 4. . Bae SE, Jung HY, Kang J.et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol. 2014;109(1):60–67.. 10.1038/ajg.2013.404) [DOI] [PubMed] [Google Scholar]
- 5. . Wang J, Xu L, Shi R.et al. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83(4):253–260.. 10.1159/000280318) [DOI] [PubMed] [Google Scholar]
- 6. . Nijevitch AA, Idrisov B, Akhmadeeva EN, Graham DY. Choosing optimal first-line Helicobacter pylori therapy: a view from a region with high rates of antibiotic resistance. Curr Pharm Des. 2014;20(28):4510–4516.. 10.2174/13816128113196660728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Kim SY, Choi DJ, Chung JW. Antibiotic treatment for Helicobacter pylori: is the end coming? World J Gastrointest Pharmacol Ther. 2015;6(4):183-198. 10.4292/wjgpt.v6.i4.183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Lee SW, Kim HJ, Kim JG. Treatment of helicobacter pylori infection in Korea: a systematic review and meta-analysis. J Korean Med Sci. 2015;30(8):1001–1009.. 10.3346/jkms.2015.30.8.1001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Redwan EM, Uversky VN, El-Fakharany EM, Al-Mehdar H. Potential lactoferrin activity against pathogenic viruses. C R Biol. 2014;337(10):581–595.. 10.1016/j.crvi.2014.08.003) [DOI] [PubMed] [Google Scholar]
- 10. . Den-Hoed CM, de-Vries AC, Mensink PB.et al. Bovine antibody-based oral immunotherapy for reduction of intragastric Helicobacter pylori colonization: a randomized clinical trial. Can J Gastroenterol. 2011;25(4):207–213.. 10.1155/2011/672093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Di-Mario F, Aragona G, Dal Bó N.et al. Bovine lactoferrin for Helicobacter pylori eradication: an open, randomized, multicenter study. Aliment Pharmacol Ther. 2006;23(8):1235–1240.. 10.1111/j.1365-2036.2006.02851.x) [DOI] [PubMed] [Google Scholar]
- 12. . Wittes J. Sample size calculations for randomized controlled trials. Epidemiol Rev. 2002;24(1):39–53.. 10.1093/epirev/24.1.39) [DOI] [PubMed] [Google Scholar]
- 13. . Di-Mario F, Aragona G, Dal Bò N.et al. Use of bovine lactoferrin for Helicobacter pylori eradication. Dig Liver Dis. 2003;35(10):706–710.. 10.1016/s1590-8658(03)00409-2) [DOI] [PubMed] [Google Scholar]
- 14. . Yuan Y, Wu Q, Cheng G.et al. Recombinant human lactoferrin enhances the efficacy of triple therapy in mice infected with Helicobacter pylori. Int J Mol Med. 2015;36(2):363–368.. 10.3892/ijmm.2015.2251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. . Canducci F, Armuzzi A, Cremonini F.et al. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14(12):1625–1629.. 10.1046/j.1365-2036.2000.00885.x) [DOI] [PubMed] [Google Scholar]
- 16. . Ciccaglione AF, Di Giulio M, Di Lodovico S.et al. Bovine lactoferrin enhances the efficacy of levofloxacin-based triple therapy as first-line treatment of Helicobacter pylori infection: an in vitro and in vivo study. J Antimicrob Chemother. 2019;74(4):1069–1077.. 10.1093/jac/dky510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. . Zou J, Dong J, Yu XF. Meta-analysis: the effect of supplementation with lactoferrin on eradication rates and adverse events during Helicobacter pylori eradication therapy. Helicobacter. 2009;14(2):119–127.. 10.1111/j.1523-5378.2009.00666.x) [DOI] [PubMed] [Google Scholar]
- 18. . Zullo A, De Francesco V, Scaccianoce G.et al. Quadruple therapy with lactoferrin for Helicobacter pylori eradication: a randomized, multicentre study. Dig Liver Dis. 2005;37(7):496–500.. 10.1016/j.dld.2005.01.017) [DOI] [PubMed] [Google Scholar]
- 19. . Dial EJ, Romero JJ, Headon DR, Lichtenberger LM, et al. Recombinant human lactoferrin is effective in the treatment of Helicobacter felis-infected mice. J Pharm Pharmacol. 2000;52(12):1541–1546.. 10.1211/0022357001777595) [DOI] [PubMed] [Google Scholar]
- 20. . Dial EJ, Hall LR, Serna H.et al. Antibiotic properties of bovine lactoferrin on Helicobacter pylori. Dig Dis Sci. 1998;43(12):2750–2756.. 10.1023/a:1026675916421) [DOI] [PubMed] [Google Scholar]
- 21. . Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417(6888):552–555.. 10.1038/417552a) [DOI] [PubMed] [Google Scholar]
- 22. . Chang JY, Shim KN, Tae CH.et al. Triple therapy versus sequential therapy for the first-line Helicobacter pylori eradication. BMC Gastroenterol. 2017;17(1):16. 10.1186/s12876-017-0579-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Eisig JN, Navarro-Rodriguez T, Teixeira AC.et al. Standard triple therapy versus sequential therapy in Helicobacter pylori eradication: a double-blind, randomized, and controlled trial. Gastroenterol Res Pract. 2015;2015:818043. 10.1155/2015/818043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372–1382.e17.. 10.1053/j.gastro.2018.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. . Johnson TJ, Hedge DD. Esomeprazole: a clinical review. Am J Health Syst Pharm. 2002;59(14):1333–1339.. 10.1093/ajhp/59.14.1333) [DOI] [PubMed] [Google Scholar]
- 26. . Gatta L, Perna F, Figura N.et al. Antimicrobial activity of esomeprazole versus omeprazole against Helicobacter pylori. J Antimicrob Chemother. 2003;51(2):439–442.. 10.1093/jac/dkg085) [DOI] [PubMed] [Google Scholar]
- 27. . Laine L, Fennerty MB, Osato M.et al. Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: results of three US multicenter, double-blind trials. Am J Gastroenterol. 2000;95(12):3393–3398.. 10.1111/j.1572-0241.2000.03349.x) [DOI] [PubMed] [Google Scholar]
- 28. . Gisbert JP, Pajares JM. Esomeprazole-based therapy in Helicobacter pylori eradication: a meta-analysis. Liv Dis. 2004;36(4):253–259.. 10.1016/j.dld.2003.12.010) [DOI] [PubMed] [Google Scholar]
- 29. . Georgopoulos SD, Ladas SD, Karatapanis S.et al. Factors that may affect treatment outcome of triple Helicobacter pylori eradication therapy with omeprazole, amoxicillin, and clarithromycin. Dig Dis Sci. 2000;45(1):63–67.. 10.1023/a:1005405209503) [DOI] [PubMed] [Google Scholar]