Figure 6.
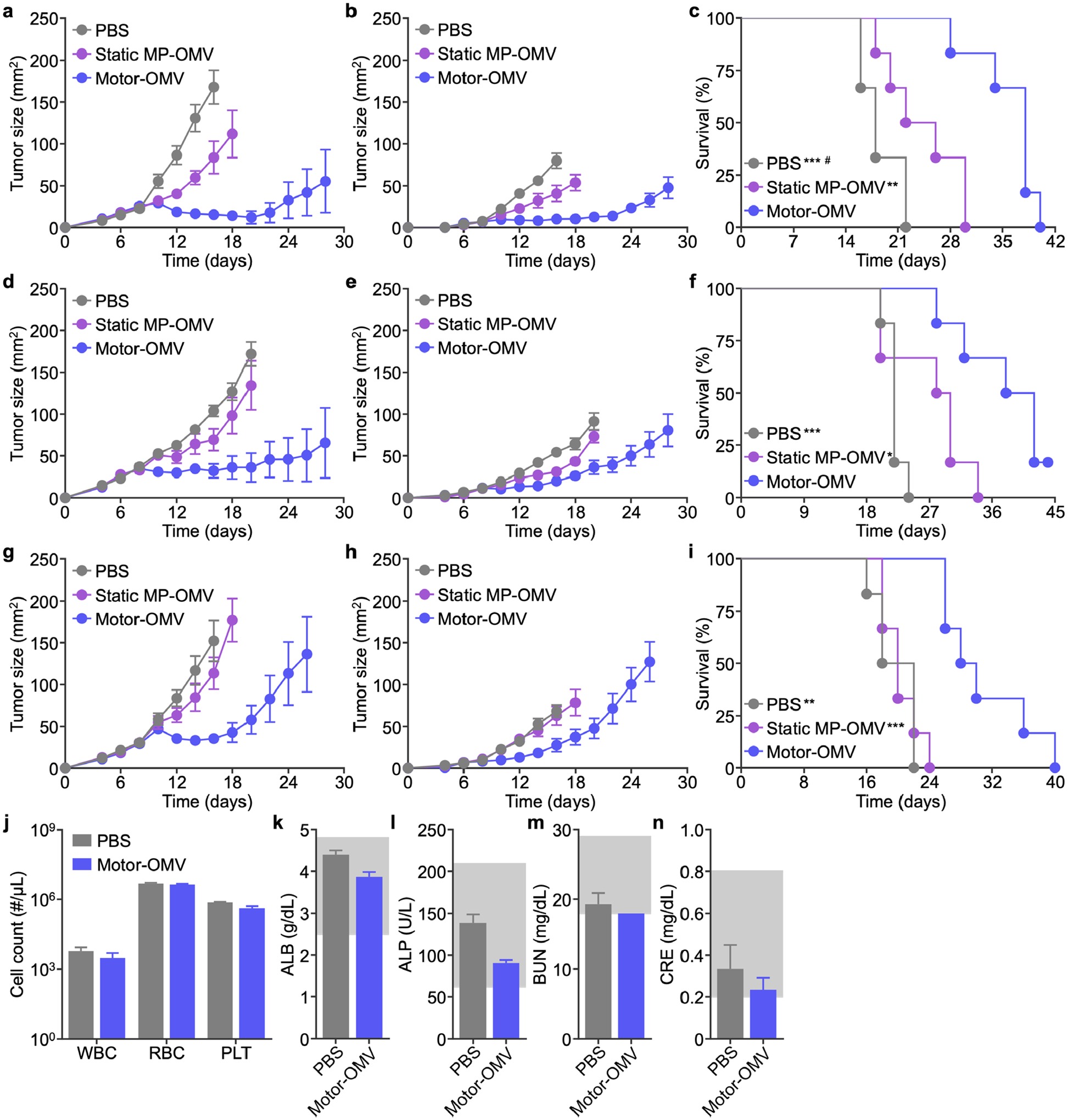
Therapeutic efficacy and safety in bilateral tumor models. a-i) MC38 (a-c), CT26 (d-f), or B16-F10 (g-i) cells were implanted subcutaneously into mice on the right flank to form a primary tumor and on the left flank at a lowered dosage to form a secondary tumor on day 0. Mice were intratumorally treated with Motor-OMV or control samples on days 8, 10, 12, and 14 at the primary tumor site. Average sizes of the primary (a, d, g) and secondary (b, e, h) tumors, as well as survival (c, f, i), were monitored over time (n = 6, mean ± SEM). j) Red blood cell (RBC), white blood cell (WBC), and platelet (PLT) counts 1 day after intratumoral treatment with Motor-OMV or PBS (n = 3, mean + SD). k-n) Levels of albumin (ALB, k), alkaline phosphatase (ALP, l), blood urea nitrogen (BUN, m), and creatinine (CRE, n) in the serum 1 day after intratumoral injection of Motor-OMV or PBS (n = 3, mean + SD). Gray boxes indicate the reference range for healthy mice. *p < 0.05, **p < 0.01, and ***p < 0.001 (compared to Motor-OMV); #p < 0.05 (compared to static MP-OMV); Mantel-Cox test.
