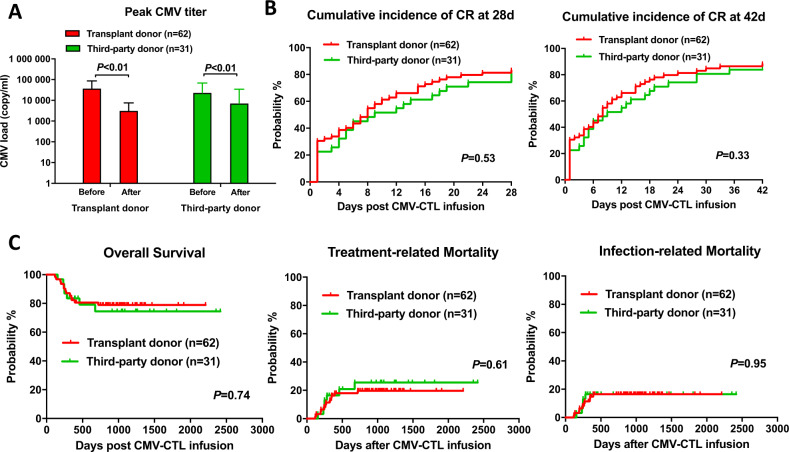
Fig. 4.
Virologic response and clinical outcomes in patients who received donor CMV-CTL and third-party CMV-CTL therapy. A Peak CMV titer change after donor and third-party CMV-CTL therapy. B Cumulative incidence of CR at 28 days and 42 days after donor and third-party CMV-CTL therapy. C Overall survival, treatment-related mortality, and infection-related mortality after donor and third-party CMV-CTL therapy. A total of 31 patients were enrolled in the third-party CMV-CTL group and matched pairs of patients (62 total) were enrolled in the donor CMV-CTL group