Abstract
Silicosis is a global occupational disease characterized by lung dysfunction, pulmonary inflammation, and fibrosis, for which there is a lack of effective drugs. Pirfenidone has been shown to exert anti-inflammatory and anti-fibrotic properties in the lung. However, whether and how pirfenidone is effective against silicosis remains unknown. Here, we evaluated the efficacy of pirfenidone in the treatment of early and advanced silicosis in an experimental mouse model and explored its potential pharmacological mechanisms. We found that pirfenidone alleviated silica-induced lung dysfunction, secretion of inflammatory cytokines (TNF-α, IL-1β, IL-6) and deposition of fibrotic proteins (collagen I and fibronectin) in both early and advanced silicosis models. Moreover, we observed that both 100 and 200 mg/kg pirfenidone can effectively treat early-stage silicosis, while 400 mg/kg was recommended for advanced silicosis. Mechanistically, antibody array and bioinformatic analysis showed that the pathways related to IL-17 secretion, including JAK-STAT pathway, Th17 differentiation, and IL-17 pathway, might be involved in the treatment of silicosis by pirfenidone. Further in vivo experiments confirmed that pirfenidone reduced the production of IL-17A induced by silica exposure via inhibiting STAT3 phosphorylation. Neutralizing IL-17A by anti-IL-17A antibody improved lung function and reduced pulmonary inflammation and fibrosis in silicosis animals. Collectively, our study has demonstrated that pirfenidone effectively ameliorated silica-induced lung dysfunction, pulmonary inflammation and fibrosis in mouse models by inhibiting the secretion of IL-17A.
Keywords: silicosis, pirfenidone, interleukin-17A, inflammation, fibrosis
Introduction
Silicosis is an occupational disease caused by long-term inhalation of silica particles, characterized by lung dysfunction, persistent pulmonary inflammation and irreversible pulmonary fibrosis [1]. Despite the efforts to reduce worker exposure to silica particles worldwide, many new silicosis cases are being reported, especially in developing countries [2]. Additionally, little progress has been made in the development of effective drugs for silicosis in recent years, with the exception of the herbal alkaloid tetrandrine, which is only approved for use in China [3]. Cough medication and bronchodilators continue to be the main therapeutic drug candidates for silicosis, even though their efficacy is limited [3]. Therefore, newer and more effective drugs are urgently needed for the treatment of silicosis.
Pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone), a synthetic small molecule derivative of pyridone [4], is one of the two recommended agents for the treatment of idiopathic pulmonary fibrosis (IPF) that has been approved in many countries [5, 6]. Multiple studies have confirmed that pirfenidone has anti-inflammatory and anti-fibrotic properties [5–8]. The drug was shown to specifically inhibit the secretion of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) [4]. Moreover, pirfenidone participates in suppressing the infiltration of inflammatory cells and activation of inflammasomes [4]. As an anti-fibrotic agent, pirfenidone can reduce the production of transforming growth factor-beta (TGF-β), which has a central role in the pathogenesis of pulmonary fibrosis [4]. Considering that these inflammatory cells and cytokines are also involved in the development of silicosis [2, 3, 9], we therefore investigated whether pirfenidone could ameliorate experimental silicosis.
Interleukin-17A (IL-17A) is an important inflammatory cytokine of the IL-17 family and is mainly secreted by a unique subset of CD4+ T cells, known as T helper 17 (Th17) cells [10, 11]. Many cytokines, such as IL-6 and TGF-β, are known to induce polarization of naïve T cells towards the Th17 phenotype, which then secrete high levels of IL-17A, resulting in the exacerbation of lung diseases including pneumonitis, asthma and pulmonary fibrosis [12, 13]. In silicosis, IL-17A is upregulated and contributes to the early inflammatory response [12, 13]. However, it remains unclear whether IL-17 is involved in other stages of silicosis. Additionally, previous studies have reported that neutralizing IL-17A reduced the accumulation of neutrophils and ameliorated pulmonary inflammation [14]. This finding suggests that by diminishing the production of IL-17A, the progression of silicosis may be potentially attenuated. However, the role of IL-17A in the treatment of silicosis requires clarification and drugs targeting IL-17A in silicosis remain to be identified.
In this study, we evaluated the efficacy of pirfenidone in mouse models of silicosis and explored its potential anti-inflammatory and anti-fibrotic mechanisms. Specifically, we administered different doses of pirfenidone to mice at early and advanced stages of silicosis. The results demonstrated that pirfenidone ameliorated silicosis by reducing the infiltration of inflammatory cells, inhibiting the production of inflammatory cytokines and the accumulation of collagen I and fibronectin. Notably, we found that 100 and 200 mg/kg of pirfenidone can effectively relieve early-stage silicosis, while a higher dose (400 mg/kg) was recommended for the treatment of advanced silicosis. We then discovered that IL-17A was upregulated during silicosis but suppressed by pirfenidone treatment. Moreover, pathways related to IL-17 secretion were associated with the action of pirfenidone. Further animal study confirmed that pirfenidone diminished silica-induced IL-17A secretion via inhibiting the phosphorylation of STAT3 in mouse lung tissues. Neutralizing IL-17A by anti-IL-17A antibody in silicosis models alleviated silica-induced lung dysfunction and pathologies. Taken together, the results of our study showed that pirfenidone was highly effective in mitigating inflammation and fibrosis associated with silicosis in mouse models, strongly suggesting that targeting IL-17A could be a viable strategy for the treatment of silicosis.
Materials and methods
Experimental animals
C57BL/6J mice (25–28 g, 10 weeks old) were purchased from the Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). Male mice were used in all experiments, as silicosis is most frequently found in male patients [15]. Therefore, all the mice we used in the experiments were male. These mice were housed in sterilized cages at 24–26 °C and 60%–70% humidity with 12/12 h dark–light cycle and given fresh water and food every week. The mouse room was maintained at specific pathogen-free conditions. All animal experimental procedures were approved by the Animal Care and Use Committee of the Peking Union Medical College.
Silica preparation and exposure
Crystalline silica (CAS7631-86-9, average particle diameter 1.6 μm, purity 99%) was purchased from Forsman Scientific Co., Ltd (Beijing, China). The particulates were made endotoxin-free by baking at 180 °C for 2 h and suspended in sterile phosphate buffer saline (PBS). The suspensions were sonicated for 30 min before use. By intratracheal instillation, silica suspensions (12 mg/mouse, 600 mg/mL, 20 µL) or PBS (20 µL) were administered to mice anesthetized with tribromoethanol (1.6 mL/100 g).
Pirfenidone treatment
Pirfenidone (PFD) were purchased from Beijing Continent Pharmaceuticals Co., Ltd (Beijing, China) and suspended in 1% carboxymethyl cellulose (CMC, 419273, Sigma-Aldrich, St. Louis, Missouri, USA). According to the dosage range of pirfenidone in clinical treatment [16–18] and in animal models [19–22], 100, 200, and 400 mg/kg pirfenidone suspensions (300 µL) were used to treat mice. Specifically, for the treatment of early silicosis, 100 and 200 mg/kg pirfenidone suspensions (300 µL) or 1% CMC (300 µL) were given to mice by daily oral gavage for 28 days, starting 1 day after exposure to silica. A total of 50 male mice were randomly divided into five groups, with 10 mice per group, designated as the PBS + Vehicle group, PBS + PFD (200 mg/kg) group, Si + Vehicle group, Si + PFD (100 mg/kg) group and Si + PFD (200 mg/kg) group. For the treatment of advanced silicosis, 200 and 400 mg/kg pirfenidone suspensions (300 µL) or 1% CMC (300 µL) were given to mice by daily oral gavage for 28 days, starting 14 days after exposure to silica. A total of 50 male mice were randomly divided into five groups, with 10 mice per group, namely the PBS + Vehicle group, PBS + PFD (400 mg/kg) group, Si + Vehicle group, Si + PFD (200 mg/kg) group and Si + PFD (400 mg/kg) group.
IL-17A neutralization
By intraperitoneal injection, 100 μg of anti-mouse IL-17A antibody (M00421-2, BOSTER, Wuhan, China) or isotype control IgG (ab18443, abcam, Cambridge, UK) was given to silica-exposed mice weekly for 4 weeks, starting 1 day after silica exposure. Pirfenidone (daily injection, 100 mg/kg, oral gavage) was given in place of and in combination with the IL-17A antibody. A total of 32 male mice were randomly divided into four groups with 8 mice per group, namely the Si + IgG group, Si + IL-17A Ab group, Si + PFD group, and Si + IL-17A Ab + PFD group.
Lung function tests
Mouse lung function was tested by a computer-controlled ventilator (FlexiVent, SCIREQ, Montreal, Quebec, Canada). Specifically, mice were anesthetized by an intraperitoneal injection of 2% pentobarbital (0.2 mL/100 g). The trachea was surgically cannulated and connected to the ventilator. Before the test, parameters were adjusted according to the weight of the mice. Subsequently, parameters of lung function were measured, including inspiratory capacity (IC), dynamic compliance (Crs), quasi-static compliance (Cst), elastance of the respiratory system (Ers), main airway resistance (Rn), and resistance of the respiratory system (Rrs).
Hemodynamic and right ventricular hypertrophy measurements
Right ventricular systolic pressure (RVSP) of mice was measured by an eight-gauge venous infusion needle connected to a pressure transducer. Data were collected with the Power Lab Data Acquisition and Analysis System (PL 3504, AD Instruments, Australia) and analyzed using the LabChart 8 software. To measure the right ventricular hypertrophy index (RVHI) of the mice, the right ventricles (RV) was separated from the left ventricle (LV) and septum (S) and weighed. RVHI was the ratio of RV weight to the sum of LV and S weight (RV/[LV + S]).
Histological staining and analysis
Left lung tissues (full pulmonary lobe) of mice were dehydrated and embedded in paraffin after fixed in 10% formalin for more than 48 h. The blocks were sliced into 5 μm sections for staining. All complete slices were scanned using the 3D HISTECH digital slice scanner.
Hematoxylin and eosin (HE) staining was performed to evaluate the pulmonary inflammation and quantified by Szapiel’s method [23]. Masson staining was used to reveal the extent of fibrosis in the lung tissues, and calculated by King’s method [24]. Specifically, all visible silicon nodules on the entire section were defined as five grades according to their different structures. Nodules without collagen deposition were defined as grade I, grade II, or grade III according to the different levels (slight, mild, severe) of inflammatory cell infiltration. Grade IV nodules were composed of collagen fibers with some inflammatory cells; Grade V nodules were composed of collagen fibers with few or no cell components. To calculate the fibrotic score (FS) of each Masson image (full pulmonary lobe), the nodules of grade I to grade V were allocated scores of 1–5. The area containing nodules of different grades (i.e., SI, SII, SIII, SIV, SV) and the total area of pulmonary lobe (St) were calculated using the Image-Pro software. The formula of fibrotic score in each image was as follows.
Sirius red staining was performed using a sirius red staining kit (G1471-2, Solarbio, Beijing, China), following the manufacturer’s instructions. Immunohistochemistry was performed to detect the expression of collagen I and IL-17A in lung tissues from the different groups. Specifically, the antigen was recovered by heating tissue sections in the citrate buffer (ZLI-9064, ORIGENE, Beijing, China). The sections were incubated with the primary antibody at 4 °C overnight and then incubated with a horseradish peroxidase polymer secondary antibody (ZLI-9018, ORIGENE, Beijing, China) at room temperature for 30 min. Finally, DAB (PV-6001, ZSGB-BIO, Beijing, China) was applied to visualize the positive areas of collagen I and AEC (ZLI-9036, ZSGB-BIO, Beijing, China) for IL-17A. The positive areas (%) of sirius red, collagen I and IL-17A staining were measured using Image-Pro Plus 6.0. See the Supplementary Table 1 for specific antibody information.
Hydroxyproline measurements
Production of hydroxyproline (HYP) in the lung tissues was tested by HYP Assay Kit (NBP2-59747, Novus Biologicals, Littleton, CO, USA) according to the manufacturer’s instructions. The absorbance of the samples was measured at 560 nm.
Leukocyte counts in BALF
Bronchoalveolar lavage fluid (BALF) was collected by entire airway lavage (0.7 mL PBS for each time, three times per mouse). The BALF was centrifuged at 1500 rpm for 5 min at 4 °C. Next, the deposited cells were resuspended in 300 µL of PBS to count the total leukocyte number by Neubauer chamber method and to count the number of lymphocytes, macrophages, and neutrophils by Giemsa staining. Based on the standard morphological characteristics of different cells, the percentages of macrophages, neutrophils, and lymphocytes in total cells were calculated.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) kits of IL-1β (MLB00C, R&D Systems, Minneapolis, MN, USA), IL-6 (M6000B, R&D Systems, Minneapolis, MN, USA) and TNF-α (MTA00B, R&D Systems, Minneapolis, MN, USA) were used to detect the inflammatory cytokines in the supernatant of BALF, following the manufacturer’s instructions.
Quantitative real-time PCR
Total messenger RNA (mRNA) was extracted from the lung tissues by using the TRIzol regent (Invitrogen, Carlsbad, CA, USA), followed by reverse transcription into complementary DNA (cDNA) by using the TIANGEN kit (KR103, Tiangen Biotechnology, Beijing, China). cDNA amplification was performed using SYBR Green I Q-PCR kit (TransGen Biotech, Beijing, China) on a Bio-Rad IQ5 system (Bio-Rad, Hercules, CA, USA). The expression of mRNA was normalized to β-actin. See Supplementary Table 2 for specific primer information.
Western blot analysis
Total protein was extracted from lung tissues using RIPA lysis buffer (P0013b, Beyotime, Shanghai, China). The concentration of the lysates was measured using a BCA Protein Assay Kit (23225, Thermo Fisher Scientific, Waltham, MA, USA). SDS–polyacrylamide gels (8 or 12%) were used to separate the proteins, which were then transferred to polyvinylidene fluoride membranes. The membranes were incubated with primary antibodies overnight at 4 °C after blocking, and then with secondary antibodies at room temperature for 1 h. The protein bands were detected by the Tanon Automatic Chemiluminescence/Fluorescence Image Analysis System (5200, Tanon, Shanghai, China) and β-actin served as the control. See Supplementary Table 3 for specific antibody information.
Antibody array
A total of 12 serum samples from different mice groups (PBS + vehicle, n = 3; Si + vehicle, n = 5; Si + 100 mg/kg PFD, n = 4) were tested in antibody array using the RayBio® L-Series Mouse Antibody Array 308 Glass Slide Kit. Serum proteins were biotinylated. Next, the glass slide arrays were blocked and the biotin labeled samples were added onto the glass slide pre-printed with antibodies, and incubated to allow for interaction with target proteins. Streptavidin-conjugated fluorescent dye (Cy3 equivalent) was applied to the array. The slides were dried and laser fluorescence scanning was used to detect the signal.
KEGG analysis
The R package clusterProfiler was used for enrichment analysis based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis [25]. Items with P value < 0.05 were considered statistically significant.
Statistical analysis
The data shown in figures were collected from independent experiments by using different mice. All data were presented as mean ± standard deviation (SD) and analyzed by using GraphPad Prism version 8.0. Shapiro–wilk test was used to detect the normality of the data. Welch’s analysis of variance (ANOVA) followed by Games–Howell’s test or Dunnett’s test were used to compare treatment groups with the control group. P < 0.05 was considered statistically significant.
Results
Low-dose pirfenidone ameliorated early-stage silicosis
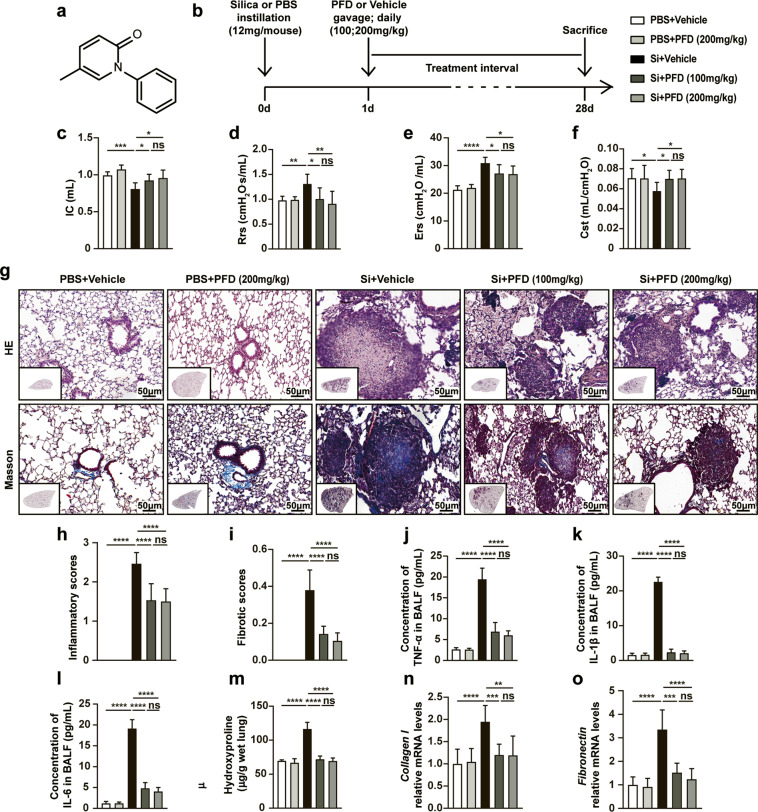
To evaluate the biological effects of pirfenidone (Fig. 1a for chemical structure) in the early stage of silicosis, we treated C57BL/6J mice with 100 or 200 mg/kg pirfenidone by intragastric gavage for 28 consecutive days after silica exposure (Fig. 1b). We subsequently evaluated the efficacy of pirfenidone in these models by testing several functional and pathophysiological parameters including lung function, right heart function, pulmonary inflammation, and fibrosis. We observed that pirfenidone treatment reduced lung dysfunction in the silica-injured mice. Specifically, lung ventilation was improved after pirfenidone treatment, indicated by increased inspiratory capacity (IC), decreased resistance (Rrs), elastance of the respiratory system (Ers) and main airway resistance (Rn) (Figs. 1c–e and S1a). Moreover, lung compliance was also improved in pirfenidone-treated mice, as indicated by increased quasi-static compliance (Cst) and dynamic compliance (Crs) (Figs. 1f and S1b). In addition, long-term exposure to silica is known to induce pulmonary hypertension characterized by increased right ventricular pressure and right ventricular hypertrophy [26]. We observed that pirfenidone administration resulted in decreased RVHI and RVSP, indicating alleviation of silica-induced pulmonary hypertension compared to the controls (Fig. S1c, d).
Fig. 1. Pirfenidone ameliorated silica-induced lung dysfunction, pulmonary inflammation and fibrosis in early-stage silicosis models.
a The chemical structure of pirfenidone. b Experimental outline. Mouse silicosis models were established by intratracheal instillation of silica suspension (12 mg/mouse, 600 mg/mL, 20 µL) or PBS (20 µL). One day after exposure to silica, pirfenidone (100 mg/kg or 200 mg/kg) was administered to mice by daily oral gavage for 28 consecutive days (n = 10 for each group). Parameters of lung function test including inspiratory capacity (IC) (c), resistance of the respiratory system (Rrs) (d), elastance of the respiratory system (Ers) (e), and quasi-static compliance (Cst) (f). Representative images of HE staining and Masson staining (g) of lung tissues from different groups. The inflammatory scores (h) were calculated based on HE staining. The fibrotic scores (i) were counted based on Masson staining. The inflammatory cytokines in BALF including TNF-α (j), IL-1β (k), and IL-6 (l) were detected by ELISA. m The contents of hydroxyproline (HYP) in lung tissues from pirfenidone or vehicle-treated mice. The mRNA expressions of collagen I (n) and fibronectin (o) in lung tissues were detected by qPCR. All data were presented as mean ± SD; n = 10 for each group; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns (no statistical significance).
According to previous reports by our group and others, acute inflammation is the primary feature of early-stage silicosis [2, 9]. We found that pirfenidone treatment attenuated silica-induced inflammation in these models through multiple lines of evidence. Histological examination by HE staining and cell counting showed that the infiltration of inflammatory cells (macrophages, neutrophils, and lymphocytes) was significantly decreased in lung tissues and BALF from pirfenidone-treated mice (Fig. 1g, h, and Fig. S2a–c). Moreover, ELISA and qPCR revealed that the production of inflammatory cytokines (TNF-α, IL-1β, and IL-6) were also diminished in BALF and lung tissues from pirfenidone-treated mice (Figs. 1j–l and S2d–f). As silicosis progresses, persistent pulmonary inflammation contributes to pulmonary fibrosis [2, 9]. Our results showed that administration of pirfenidone in the early stage of silicosis led to diminished formation of silicon nodules and deposition of collagen fibers (Figs. 1g, i and S3a, b). Moreover, the reduction in fibrosis associated with pirfenidone was also demonstrated by decreased HYP, collagen I, and fibronectin in the treated mice compared to untreated controls (Figs. 1m–o and S3c, d).
Taken together, these results indicated that administration of pirfenidone in the early stage of silicosis appeared to alleviate silica-induced lung dysfunction, right heart dysfunction, pulmonary inflammation and fibrosis. Notably, we found no detectable difference in the protective effects between 100 and 200 mg/kg, suggesting that these doses of pirfenidone can effectively ameliorate silicosis progression in its early stage.
Pirfenidone inhibited the secretion of IL-17A in early-stage silicosis
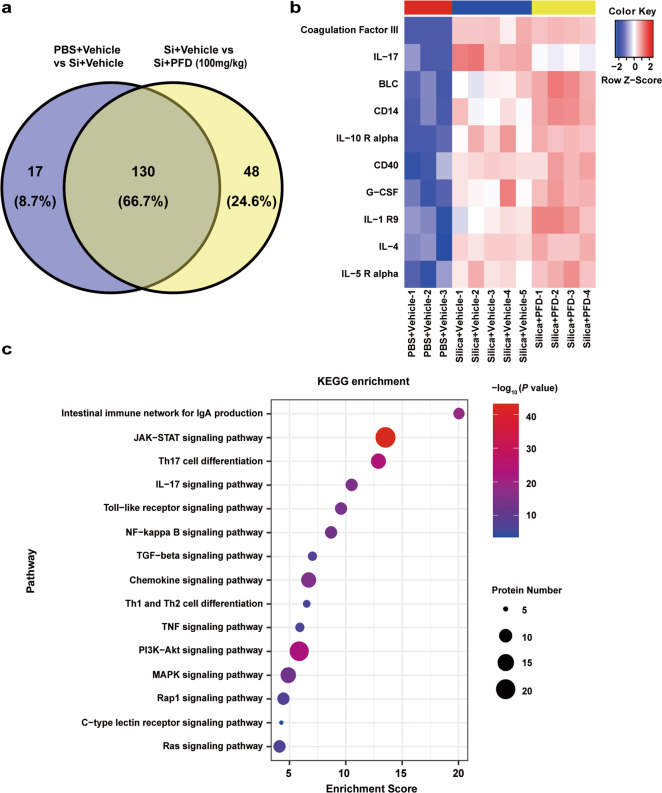
To further investigate the potential mechanisms underlying pirfenidone-mediated alleviation of early-stage silicosis, we collected serum samples from mice in each treatment group for antibody array. We found 130 overlapping differently regulated proteins related to both silica exposure and pirfenidone treatment (Fig. 2a). Among these proteins, IL-17A was increased after silica exposure, but was diminished in the pirfenidone treatment group (Fig. 2b). We further confirmed the relevance of these proteins to pirfenidone by using KEGG analysis to determine which pathways may be involved in the treatment of silicosis with pirfenidone. The results confirmed that the IL-17A signaling pathway was significantly enriched, in addition to other pathways (JAK-STAT signaling pathway and Th17 cell differentiation) known to be associated with IL-17A secretion (Fig. 2c). In light of these findings, we hypothesized that pirfenidone alleviated silicosis by reducing the secretion of IL-17A.
Fig. 2. Bioinformatic analysis for the antibody array.
a Venn diagram shows that the 147 serum proteins were related to silica exposure and 178 serum proteins were related to pirfenidone treatment, with 130 overlapping differentially regulated proteins related to both silica exposure and pirfenidone treatment (n = 3 for PBS + Vehicle; n = 5 for Si + Vehicle; n = 4 for Si + 100 mg/kg PFD). b Heatmap shows expression of the top 10 proteins in the group of 130 proteins (ranked by P value). c Functional enrichment analysis of the 130 proteins based on KEGG.
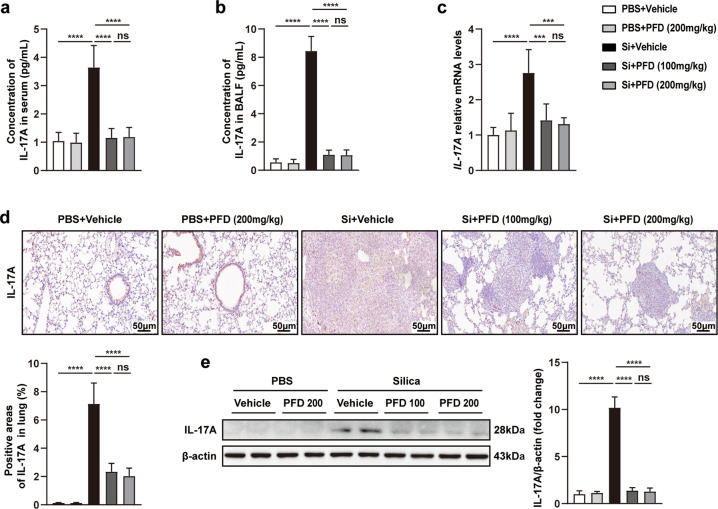
To test this hypothesis, we detected the expression of IL-17A in serum, BALF, and lung tissues from pirfenidone-treated silicosis mice. As expected, compared with vehicle-treated mice, the levels of IL-17A were significantly lower in the serum and BALF of pirfenidone-treated mice (Fig. 3a, b). Moreover, qPCR, immunohistochemistry, and Western blot showed that pirfenidone treatment was associated with reduced IL-17A transcription and protein expression in lung tissues (Fig. 3c–e). Together, these results suggested that pirfenidone inhibited the secretion of IL-17A in the early stage of silicosis. Interestingly, we again noted that there was no difference in IL-17A expression between low and high pirfenidone dosage groups, indicating that these doses of pirfenidone were adequate to reduce the production of IL-17A in the early stage of silicosis.
Fig. 3. Pirfenidone inhibited the secretion of IL-17A in early-stage silicosis models.
ELISA was used to detect the protein levels of IL-17A in serum (a) and BALF (b). The mRNA and protein levels of IL-17A in lung tissues from different groups were measured by qPCR (c), immunohistochemistry (d), and Western blot analysis (e). All data were presented as mean ± SD; n = 10 for each group; ***P < 0.001, ****P < 0.0001, ns (no statistical significance).
Many investigators have confirmed that inhibiting the phosphorylation of STAT3 can diminish the differentiation of CD4+ T cells into Th17 cells, resulting in lowered IL-17 secretion [27]. Our results also suggested that the JAK-STAT signaling pathway may contribute to the therapeutic effects of pirfenidone (Fig. 2c). Therefore, to explore whether the JAK-STAT pathway was directly modulated by pirfenidone, we performed Western blot analysis to detect the phosphorylated STAT3 and total STAT3 in lung tissues of mouse experimental models. The data showed that administration of pirfenidone in the early stage of silicosis inhibited the phosphorylation of STAT3 at Tyr 705 and this was associated with reduced protein levels of IL-17A (Fig. S4a–d). Moreover, we also observed no detectable difference in phosphorylation of STAT3 between 100 and 200 mg/kg pirfenidone in early-stage silicosis. Collectively, our data provided evidence suggesting that pirfenidone reduced the production of IL-17A in mouse lung tissues by inhibiting the activation of the JAK-STAT signaling pathway.
Neutralizing IL-17A alleviated pulmonary pathologies in early-stage silicosis
To further explore whether inhibition of IL-17A can delay the progression of early silicosis, we treated silicosis mice with a control IgG antibody, IL-17A antibody, 100 mg/kg pirfenidone or a combination of anti-IL-17A and pirfenidone (Fig. S5a). To confirm successful IL-17A neutralization, we measured IL-17A levels in the BALF and serum by ELISA. The results showed that IL-17A levels in IL-17A antibody- and pirfenidone-treated groups were significantly lower than the IgG antibody-treated group, suggesting that IL-17A was blocked by the IL-17A antibody and pirfenidone (Fig. S5b, c).
Next, we performed functional tests and molecular experiments to evaluate the treatment efficacy of the IL-17A antibody in silicosis. Lung function test showed that neutralizing IL-17A improved lung ventilation (Fig. S5d–g) and compliance (Fig. S5h, i) in early silicosis models. Moreover, silica-induced pulmonary hypertension was also ameliorated, indicated by decreased RVHI and RVSP in IL-17A antibody-treated groups (Fig. S5j, k). In addition, HE staining (Fig. S6a, b), qPCR (Fig. S6c–e), and ELISA (Fig. S6f–h) together revealed that the mRNA and protein levels of inflammatory cytokines (TNF-α, IL-1β, IL-6) in the anti-IL-17A group were significantly lower than those of the IgG control group, indicating that IL-17A neutralization attenuated silica-induced pulmonary inflammation in these models. Furthermore, neutralizing IL-17A alleviated silica-induced pulmonary fibrosis in these models, indicated by decreased fibrotic scores (Fig. S7a, b), collagen I deposition (Fig. S7a, c), and HYP content (Fig. S7d). Decreased mRNA and protein levels of collagen I and fibronectin in IL-17A antibody-treated groups further supported the anti-fibrotic effects (Fig. S7e–h). Together, these results suggested that neutralizing IL-17A effectively alleviated silicosis-related pathologies. Interestingly, we also observed that the treatment efficacy of pirfenidone was better than that of the IL-17A neutralizing antibody, but equivalent to combination of pirfenidone and IL-17A antibody, suggesting that IL-17A is one of the main targets of pirfenidone in the treatment of silicosis, but other molecule targets may have been involved.
High-dose pirfenidone ameliorated advanced silicosis
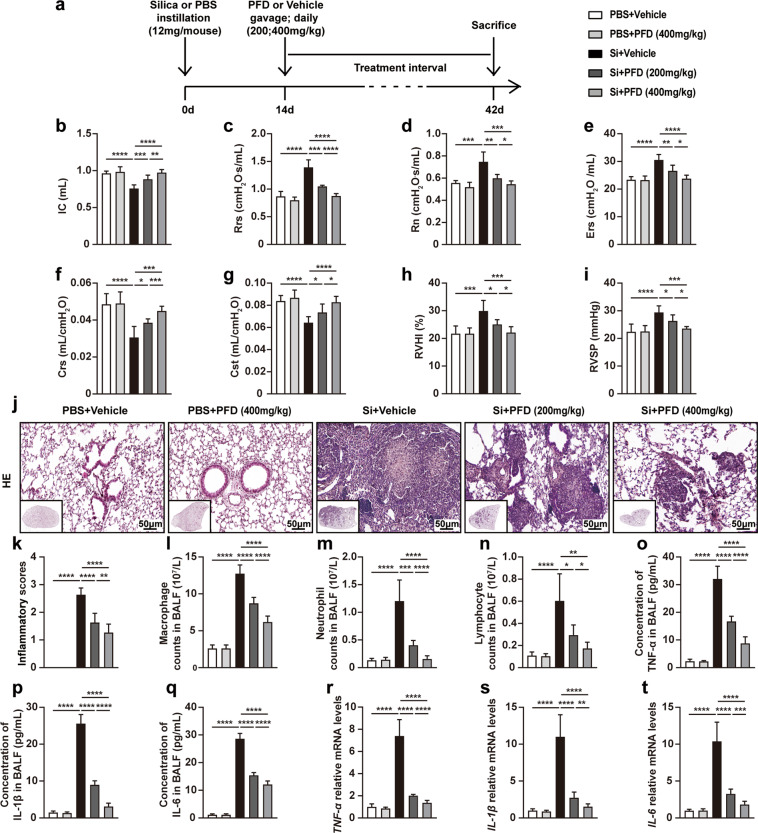
Although we initially demonstrated that a low dose of pirfenidone could ameliorate silicosis in its early stage, there are, unfortunately, no reliable diagnostic strategies currently available for silicosis in this stage of its progression. Clinically confirmed silicosis patients are always accompanied with apparent pulmonary fibrosis, characteristic of the advanced stage [1]. Therefore, we further explored whether pirfenidone was able to ameliorate silicosis in the advanced stage. To this end, we administered pirfenidone at 200 or 400 mg/kg doses to mice 14 days after of silica exposure to observe whether pirfenidone affected advanced silicosis in a dose-dependent manner (Fig. 4a). We evaluated the efficacy of these treatments by measuring the same functional and pathophysiological parameters as we did for the early-stage experiments.
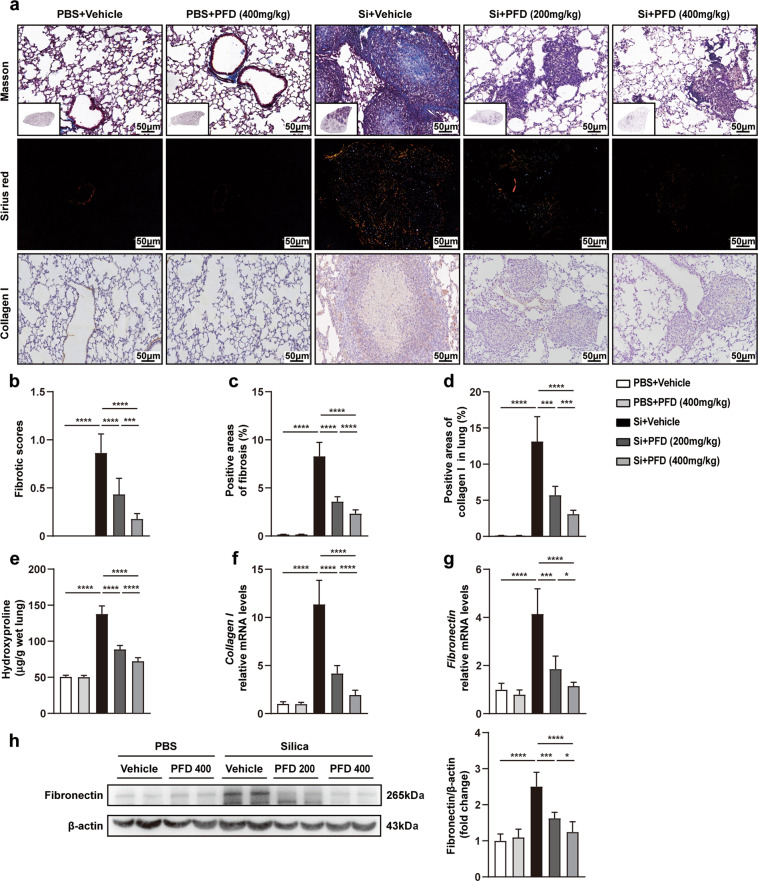
Fig. 4. High-dose pirfenidone was more effective in alleviating silica-induced lung dysfunction, right heart dysfunction, and pulmonary inflammation in advanced silicosis models.
a Experimental outline. Mouse silicosis models were established by intratracheal instillation of silica suspension (12 mg/mouse, 600 mg/mL, 20 µL) or PBS (20 µL). Two weeks after silica exposure, pirfenidone (200 or 400 mg/kg) was administered to mice by daily oral gavage for 28 consecutive days (n = 10 for each group). Lung function of different groups was evaluated by inspiratory capacity (IC) (b), resistance of respiratory system (Rrs) (c), main airway resistance (Rn) (d), elastic resistance of respiratory system (Ers) (e), dynamic compliance (Crs) (f) and quasi-static compliance (Cst) (g). Right heart function of different groups was evaluated by right ventricular hypertrophy index (RVHI) (h) and right ventricular systolic pressure (RVSP) (i). Representative images of HE staining (j) in lung tissues from different groups. The inflammatory scores (k) were calculated based on HE staining. The cell counts of macrophage (l), neutrophil (m), and lymphocyte (n) in BALF from different groups were calculated after Giemsa staining. ELISA was used to detect the protein levels of inflammatory cytokines in BALF including TNF-α (o), IL-1β (p), and IL-6 (q). qPCR was used to detect the mRNA levels of inflammatory cytokines in lung tissues including TNF-α (r), IL-1β (s), and IL-6 (t). All data were expressed as mean ± SD; n = 10 for each group; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Lung function tests showed that pirfenidone improved lung ventilation (Fig. 4b–e) and compliance (Fig. 4f, g). Similarly, compared with vehicle-treated silicosis models, pirfenidone attenuated silica-induced right ventricular hypertrophy and pulmonary hypertension (Fig. 4h, i). In addition, HE staining, inflammatory cell counting, ELISA, and qPCR showed that the infiltration of inflammatory cell and the accumulation of inflammatory cytokines were significantly reduced by low- and high-dose pirfenidone compared to the untreated controls (Fig. 4j–t). Masson staining, Sirius red staining, and HYP measurement revealed that collagen content was also diminished by pirfenidone treatment (Fig. 5a–c, e). Consistent with these findings, decreased collagen I and fibronectin expression in silicosis lung tissues supported pirfenidone-mediated inhibition of pulmonary fibrosis (Fig. 5a, d, f–h). Together, these results suggested that pirfenidone administration improved lung function and reduced or delayed pulmonary inflammation and fibrosis in silicosis, even after the appearance of fibrotic lesions. Notably, we found that 400 mg/kg pirfenidone was more effective than 200 mg/kg, indicating that high-dose pirfenidone might be more suitable for the treatment of advanced stage silicosis.
Fig. 5. High dose of pirfenidone was more effective in ameliorating silica-induced pulmonary fibrosis in advanced silicosis.
Masson staining, Sirius red staining and immunohistochemistry (a) were performed in mouse lung tissues to evaluate the levels of pulmonary fibrosis. The fibrotic scores (b) were calculated based on Masson staining. The positive areas of fibrosis (c) were calculated based on Sirius red staining. The positive areas of collagen I (d) were calculated based on immunohistochemistry. The contents of hydroxyproline (e) in lung tissues were detected by an HYP kit. The mRNA expression levels of collagen I (f) and fibronectin (g) were tested by qPCR. The protein levels of fibronectin (h) were detected by Western blot analysis. All data were presented as mean ± SD; n = 10 for each group; *P < 0.05, ***P < 0.001, ****P < 0.0001.
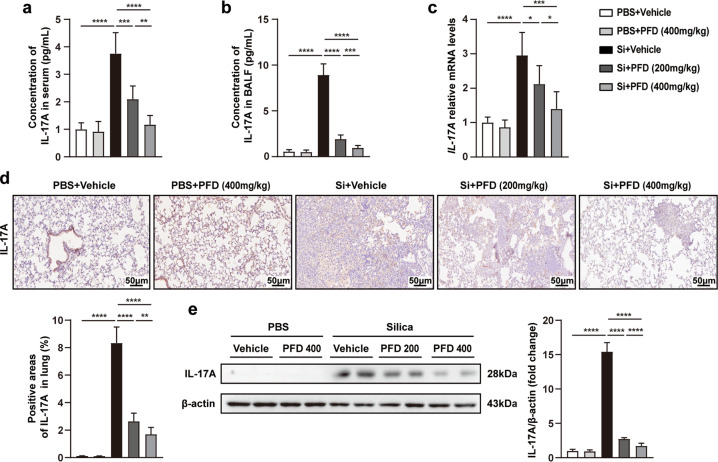
Pirfenidone inhibited the secretion of IL-17A in advanced stage silicosis
To further explore whether pirfenidone ameliorated advanced silicosis via inhibition of IL-17A production, we firstly performed ELISA to measure the protein levels of IL-17A in the BALF and serum samples of mice exposed to silica with different duration (1 day, 3 days, 7 days, 14 days, 28 days and 42 days). Our data showed that, compared with PBS-treated group, the production of IL-17A in BALF and serum increased significantly from day 1 to day 7 (Fig. S8a, b). Although IL-17A decreased from the day 7 to day 42, the protein levels of IL-17A in serum and BALF were still higher than that of the control group until day 42, indicating that IL-17A remained at a high level in advanced silicosis. Therefore, we speculated that pirfenidone could also inhibit the secretion of IL-17A in advanced silicosis models.
We measured IL-17A mRNA and protein expression in mice treated with pirfenidone. Quantification by ELISA showed that IL-17A levels in serum and BALF were significantly reduced among pirfenidone-treated mice (Fig. 6a, b). Moreover, compared with vehicle-treated mice, the expression of IL-17A was also decreased in lung tissues, confirmed by qPCR, immunohistochemistry, and Western blot analyses (Fig. 6c–e). We again noted that, compared with 200 mg/kg pirfenidone, 400 mg/kg was more effective in inhibiting the production of IL-17A. Taken together, these data indicated that pirfenidone treatment inhibited IL-17A secretion in advanced silicosis.
Fig. 6. Pirfenidone diminished the production of IL-17A in advanced silicosis models.
ELISA was performed to measure the protein expression of IL-17A in serum (a) and BALF (b) from different groups. The mRNA and protein levels of IL-17A in lung tissues were measured by qPCR (c), immunohistochemistry (d) and Western blot analysis (e), respectively. All data were presented as mean ± SD; n = 10 for each group; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To further explore whether pirfenidone reduced the secretion of IL-17A via inhibiting STAT3 phosphorylation, we performed Western blot analysis to assess the protein expression of phosphorylated and total STAT3 in mouse lung tissues. The data showed that administration of pirfenidone in advanced stage silicosis inhibited silica-induced phosphorylation of STAT3, associated with reduced protein levels of IL-17A (Fig. S9a–d). Moreover, we also found that 400 mg/kg pirfenidone was more effective than 200 mg/kg in inhibiting STAT3 phosphorylation. Together, these data indicated that pirfenidone reduced the production of IL-17A in advanced silicosis models by inhibiting the phosphorylation of STAT3.
Discussion
In this study, we found that pirfenidone inhibited the secretion of IL-17A and ameliorated silicosis. Specifically, both 100 and 200 mg/kg of pirfenidone can alleviate silica-induced lung dysfunction, infiltration of inflammatory cells, production of inflammatory cytokines and the accumulation of collagen I and fibronectin in the early stage of silicosis. Higher doses of pirfenidone (400 mg/kg) more effectively induced these therapeutic effects during advanced silicosis. At the molecular level, we found that pirfenidone resulted in reduced production of IL-17A in both early and advanced silicosis models via inhibiting STAT3 phosphorylation.
Multiple clinical trials and basic research studies have confirmed that pirfenidone can safely inhibit pulmonary inflammation and fibrosis in IPF [5, 7]. One study reported that administration of pirfenidone on the day after silica exposure could effectively inhibit the progression of pulmonary inflammation and fibrosis in rat silicosis models [28]. However, in mouse silicosis models, it has remained unclear whether pirfenidone could also ameliorate the progression of silicosis, especially in its advanced stage. In our study, we demonstrate that pirfenidone can alleviate silica-induced lung dysfunction, right heart dysfunction, pulmonary inflammation and fibrosis in both the early and advanced stage of silicosis. Interestingly, we found no difference between 100 and 200 mg/kg treatments for early-stage silicosis, although 400 mg/kg pirfenidone was more effective for the treatment of advanced silicosis. These observations suggest that lower pirfenidone dosage may be more suitable for silicosis patients without pulmonary fibrosis, while higher doses are required after the occurrence of pulmonary fibrosis in silicosis patients. More importantly, our findings with advanced silicosis are more relevant to clinical practice, as silicosis patients are mostly diagnosed with advanced disease. Our rescue experiments, where the treatment was initiated 2 weeks after silica exposure, were more informative than the prophylactic experiments in confirming the efficacy of pirfenidone and its potential for translation.
IL-17A is generally regarded as an inflammatory cytokine, which promotes inflammation in damaged tissues [11]. One study reported that IL-17A production was upregulated in silicosis lung tissues, which then contributed to pulmonary inflammation [14]. Our results demonstrate that IL-17A is increased not only in the early stage but also in the advanced stage of silicosis. Moreover, studies have reported that inhibition of IL-17A (using IL-17R−/− mice or IL-17A antibody) alleviated pulmonary inflammation caused by neutrophil infiltration in early-stage silicosis [14, 29]. Consistently, our data confirmed that neutralizing IL-17A can delay the progression of silica-induced silicosis. These observations further confirm the role of IL-17A in promoting the deterioration of silicosis. More importantly, we also demonstrated pirfenidone can inhibit IL-17A secretion in both early and advanced silicosis models, which was associated with the amelioration of silica-induced pulmonary inflammation and fibrosis. These results together indicated that pirfenidone may be an effective candidate drug for the inhibition of IL-17A signaling in silicosis.
In our study, several pathophysiological and molecular experiments demonstrated that pirfenidone inhibited IL-17A secretion in silica-induced mouse models. However, the complete molecular mechanisms underlying this inhibitory effects are unknown. Studies have reported that IL-17A is mainly secreted by Th17 cells, and upregulated cytokines including IL-6 and TGF-β, which are induced by silica exposure, can also promote the differentiation of Th17 cells via activating the JAK-STAT pathway by promoting STAT3 phosphorylation [27, 30, 31]. Similarly, in our study, KEGG enrichment analysis of the antibody array suggested that the JAK-STAT pathway and Th17 cell differentiation were involved in the treatment of silicosis with pirfenidone (Fig. 2c). Moreover, pirfenidone inhibited silica-induced IL-6 secretion (Figs. 1l, 4q, t and S2f) and STAT3 phosphorylation (Figs. S4 and S9) in mouse lung tissues. Based on the existing studies and our results, we speculate that pirfenidone inhibits silica-induced cytokines secretion, including IL-6 and TGF-β, which then reduce the differentiation of Th17 cells and IL-17A secretion via inhibiting STAT3 phosphorylation. The decreased levels of IL-17A alleviated silica-induced inflammatory response, resulting in the amelioration of silicosis. However, these mechanisms require considerably more experimental data to clarify the full network of interactions. In addition, our data also revealed that the efficacy of pirfenidone was better than that of the IL-17A neutralizing antibody, but equivalent to combination of pirfenidone and IL-17A antibody, suggesting that pirfenidone may target additional molecules to treat silicosis. Additional specific mechanisms mediated by pirfenidone in silicosis need to be further studied.
In summary, silicosis is a global occupational disease lacking effective drugs for treatment. Here, we show that pirfenidone ameliorates silicosis regardless of disease stage. Furthermore, pirfenidone-mediated inhibition of IL-17A production represents an important mechanism of action. Based on these findings, we anticipate that IL-17A may be a novel and effective target for the treatment of silicosis, and pirfenidone may represent breakthrough drug treatment for silicosis patients.
Supplementary information
Acknowledgements
This work was financially supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences [grant number: 2018-12M-1-001] (to CW), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [grant number: 2018RC31001] (to CW) and the National Natural Science Foundation of China (91739107) (to JW).
Author contributions
ZJC designed the project, performed experiments, analyzed data and drafted the manuscript. YL designed the project, performed experiments and analyzed data. ZZ, ZGL, MYS, XMQ, BCL, and XRZ contributed to the construction of silicosis models and helped collect experimental samples. JLP and ZFH performed bioinformatic analysis. PRY, HPD, JW, and CW helped design the project, commented on the manuscript and supervised all aspects of the project.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Zhu-jie Cao, Ying Liu
Contributor Information
Hua-ping Dai, Email: daihuaping@ccmu.edu.cn.
Jing Wang, Email: wangjing@ibms.pumc.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00706-4.
References
- 1.Leung CC, Yu IT, Chen W. Silicosis. Lancet. 2012;379:2008–18. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 2.Barnes H, Goh N, Leong TL, Hoy R. Silica-associated lung disease: an old-world exposure in modern industries. Respirology. 2019;24:1165–75.. doi: 10.1111/resp.13695. [DOI] [PubMed] [Google Scholar]
- 3.Lopes-Pacheco M, Bandeira E, Morales MM. Cell-based therapy for silicosis. Stem Cells Int. 2016;2016:50918–38.. doi: 10.1155/2016/5091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruwanpura SM, Thomas BJ, Bardin PG. Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am J Respir Cell Mol Biol. 2020;62:413–22. doi: 10.1165/rcmb.2019-0328TR. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 6.Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26:146. doi: 10.1183/16000617.0057-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George PM, Wells AU. Pirfenidone for the treatment of idiopathic pulmonary fibrosis. Expert Rev Clin Pharmacol. 2017;10:483–91.. doi: 10.1080/17512433.2017.1295846. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-de LMD, Sanchez-Roque C, Montoya-Buelna M, Sanchez-Enriquez S, Lucano-Landeros S, Macias-Barragan J, et al. Role and new insights of pirfenidone in fibrotic diseases. Int J Med Sci. 2015;12:840–7. doi: 10.7150/ijms.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z, Song M, Liu Y, Pang J, Li Z, Qi X, et al. A novel pathophysiological classification of silicosis models provides some new insights into the progression of the disease. Ecotoxicol Environ Saf. 2020;202:1108–34.. doi: 10.1016/j.ecoenv.2020.110834. [DOI] [PubMed] [Google Scholar]
- 10.Kurschus FC, Moos S. IL-17 for therapy. J Dermatol Sci. 2017;87:221–7.. doi: 10.1016/j.jdermsci.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Gurczynski SJ, Moore BB. IL-17 in the lung: the good, the bad, and the ugly. Am J Physiol Lung Cell Mol Physiol. 2018;314:L6–16. doi: 10.1152/ajplung.00344.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 13.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64:477–85. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo RS, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, et al. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 2010;184:6367–77. doi: 10.4049/jimmunol.0900459. [DOI] [PubMed] [Google Scholar]
- 15.Pollard KM. Silica, silicosis, and autoimmunity. Front Immunol. 2016;7:97. doi: 10.3389/fimmu.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 17.Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:756–61.. doi: 10.1164/rccm.201701-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2018;197:356–63.. doi: 10.1164/rccm.201706-1301OC. [DOI] [PubMed] [Google Scholar]
- 19.Inomata M, Kamio K, Azuma A, Matsuda K, Kokuho N, Miura Y, et al. Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis. Respir Res. 2014;15:16. doi: 10.1186/1465-9921-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Li H, Liu S, Pan P, Su X, Tan H, et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol Immunol. 2018;99:134–44.. doi: 10.1016/j.molimm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Shi G. Pirfenidone activates cannabinoid receptor 2 in a mouse model of bleomycin-induced pulmonary fibrosis. Exp Ther Med. 2019;18:4241–8.. doi: 10.3892/etm.2019.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleh MA, Antar SA, Hazem RM, El-Azab MF. Pirfenidone and vitamin D ameliorate cardiac fibrosis induced by doxorubicin in Ehrlich ascites carcinoma bearing mice: modulation of monocyte chemoattractant protein-1 and Jun N-terminal kinase-1 pathways. Pharmaceuticals. 2020;13:11. doi: 10.3390/ph13110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–99. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- 24.King EJ. Silicosis. Lect Sci Basis Med. 1952;2:108–38. [PubMed] [Google Scholar]
- 25.Lawrence M, Huber W, Pages H, Aboyoun P, Carlson M, Gentleman R, et al. Software for computing and annotating genomic ranges. PLoS Comput Biol. 2013;9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omland O, Wurtz ET, Aasen TB, Blanc P, Brisman JB, Miller MR, et al. Occupational chronic obstructive pulmonary disease: a systematic literature review. Scand J Work Environ Health. 2014;40:19–35. doi: 10.5271/sjweh.3400. [DOI] [PubMed] [Google Scholar]
- 27.Camporeale A. Poli VIL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front Biosci. 2012;17:2306–26. doi: 10.2741/4054. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Yang Z, Jia Q, Bo C, Shao H, Zhang Z. Pirfenidone inhibits epithelial-mesenchymal transition and pulmonary fibrosis in the rat silicosis model. Toxicol Lett. 2019;300:59–66. doi: 10.1016/j.toxlet.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Li C, Weng D, Song L, Tang W, Dai W, et al. Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice. Toxicol Appl Pharmacol. 2014;275:62–72. doi: 10.1016/j.taap.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–94. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed S, Misra DP, Agarwal V. Interleukin-17 pathways in systemic sclerosis-associated fibrosis. Rheumatol Int. 2019;39:1135–43.. doi: 10.1007/s00296-019-04317-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.