Figure 3. Mechanisms of fatty acid (FA)-dependent H+ transport by uncoupling protein 1 (UCP1) and ADP/ATP carrier (AAC).
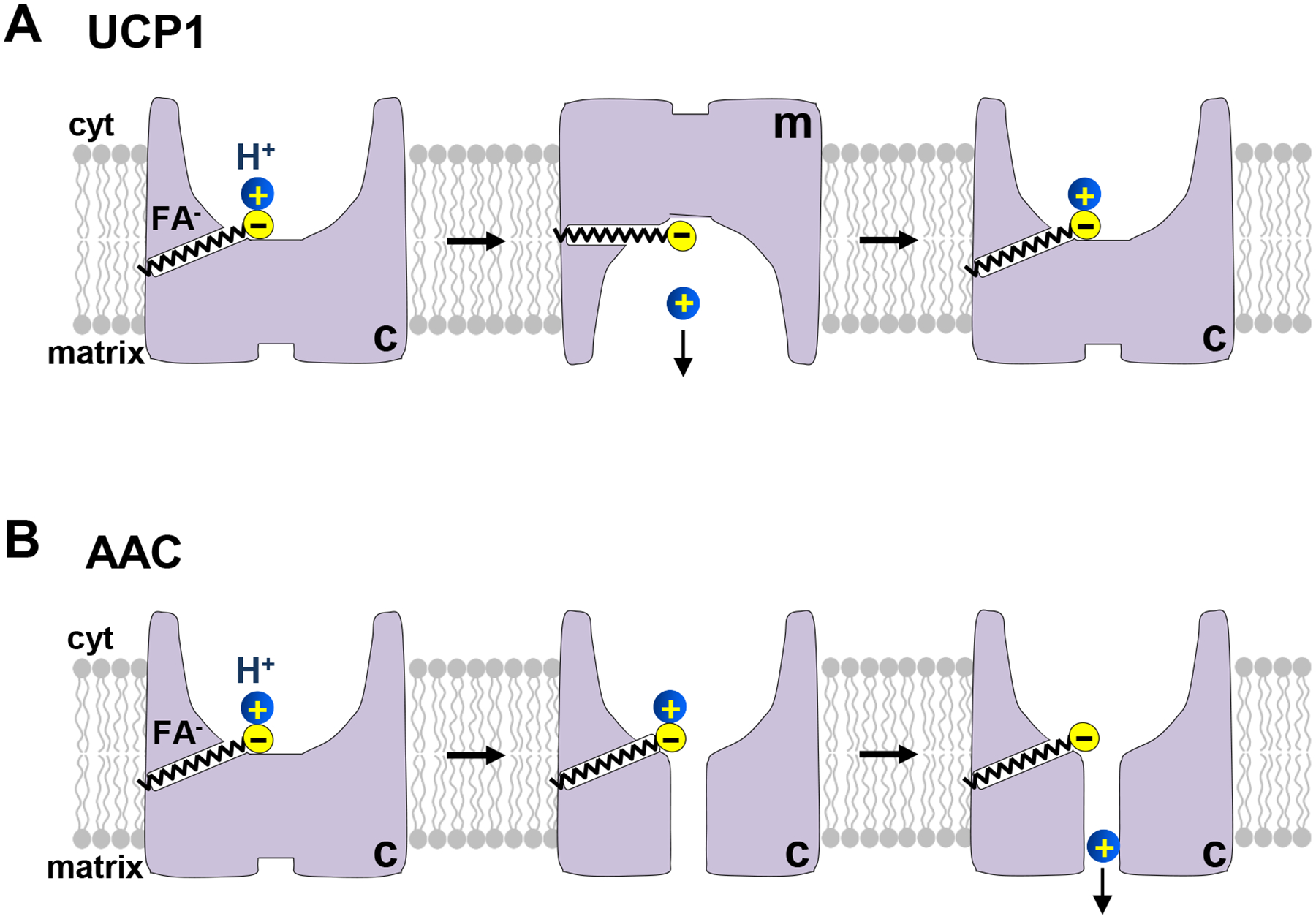
(A) The mechanism of H+ leak via UCP1. UCP1 functions as a FA anion/H+ symporter. FA are UCP1 transport substrates and induce UCP1 transition between the c- and m-state. When long-chain FA and H+ are bound, UCP1 undergoes conformation changes between the c- and m-state, enabling H+ translocation. However, long-chain FA do not dissociate from UCP1 because they are “caught” within UCP1 by hydrophobic interactions. Thus, each long-chain FA can facilitate transport of multiple H+. (B) The mechanism of H+ leak via AAC. In contrast to UCP1, AAC does not transport FA anions, and FA cannot induce AAC transition between the c- and m-state. Instead, binding of a long-chain FA anion to AAC might induce a minor conformational change to open a narrow translocation pathway for H+ across AAC. In addition, long-chain FA provide an essential binding site for H+ permeation through AAC.
