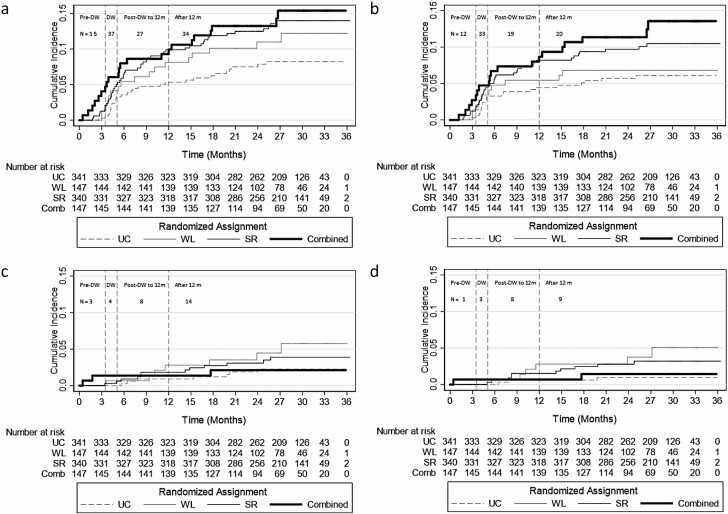
Figure 2.
Cumulative incidence curves portraying risk of (a) any adverse events, (b) orthostatic symptoms, (c) falls, fracture, or syncope, or (d) falls with the number at risk according to the 4 randomized assignments over the 36-month trial surveillance period: usual care (UC), weight loss (WL), sodium reduction (SR), or combined weight loss and sodium reduction (Combined). Participants were censored if they reported an event or administratively (as a result of developing the primary outcome in the study or at 36 months). N is the number of recurrent events reported during each study phase. Pre-drug withdrawal (DW) is the study period after randomization and up to and including the visit initiating DW. DW includes all DW visits after drug withdrawal began up to and including the 12-month visit. In the event that study phases were not clearly labeled (6 orthostatic symptoms or adverse events, 2 orthostatic symptoms, 4 adverse events, 3 falls), we relied on the mean follow-time for first DW (3.5 months) and last DW (5.1 months).