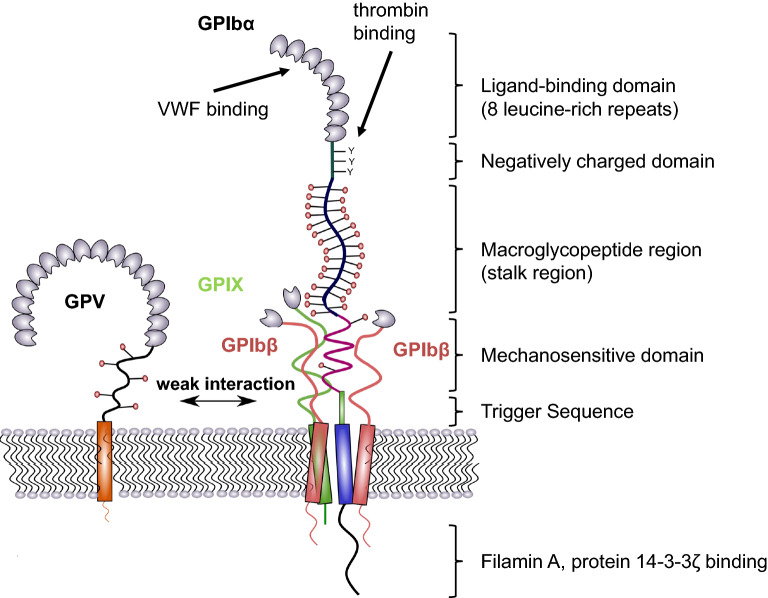
Fig. 1.
Structure of GPIb-IX. The largest subunit of the complex is GPIbα, which consists of a N-terminal binding domain (made up of 8 leucine-rich repeats), a negatively charged domain, a sialomucin region (macroglycopeptide domain), the mechanosensitive domain (MSD), and proximal to the membrane, the trigger sequence. Intracellularly, GPIbα interacts with protein 14-3-3ζ and filamin A which links the complex to the cytoskeleton. The transmembrane domain provides a close interaction with GPIbβ and GPIX to form a stable parallel four-helical bundle structure. This complex structure is further strengthened by the interplay of GPIbβ and GPIX extracellular domains with the MSD in GPIbα. GPV is weakly associated with GPIb-IX by polar interactions and can be replaced by other receptors