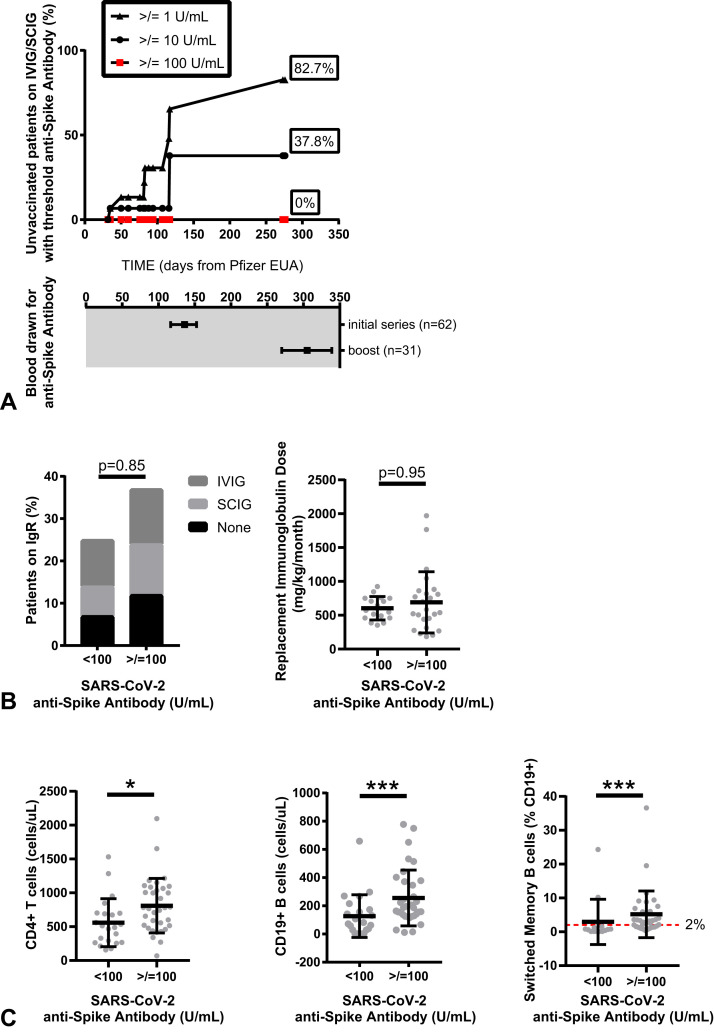
Figure 4.
Evaluation of timing of SARS-CoV-2 anti-spike antibody testing in relation to potential for passive antibody transfer (A). Detection of threshold anti-spike antibodies (greater than 1 U/mL, greater than 10 U/mL, or greater than 100 U/mL) in vaccine-naive predominant antibody deficiency (PAD) patients receiving intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG) therapy. Data are shown as percent positive by Kaplan-Meier curve; symbols indicate events over 276 days after emergency use authorization (EUA) of the Pfizer vaccine (top) with corresponding dates of blood draw for all PAD patients included in this study shown as median (± interquartile range) days (bottom). Threshold vaccine response, defined in the study as an anti-spike antibody level of 100 U/mL or greater, is shown in relation to immunoglobulin replacement therapy (B) and underlying immunophenotype (C). Symbols represent unique individuals; bars represent means (±SD) of total indicated patients (n). ∗P < .05; ∗∗∗P < .001. IgR, Immunoglobulin replacement