Figure 4:
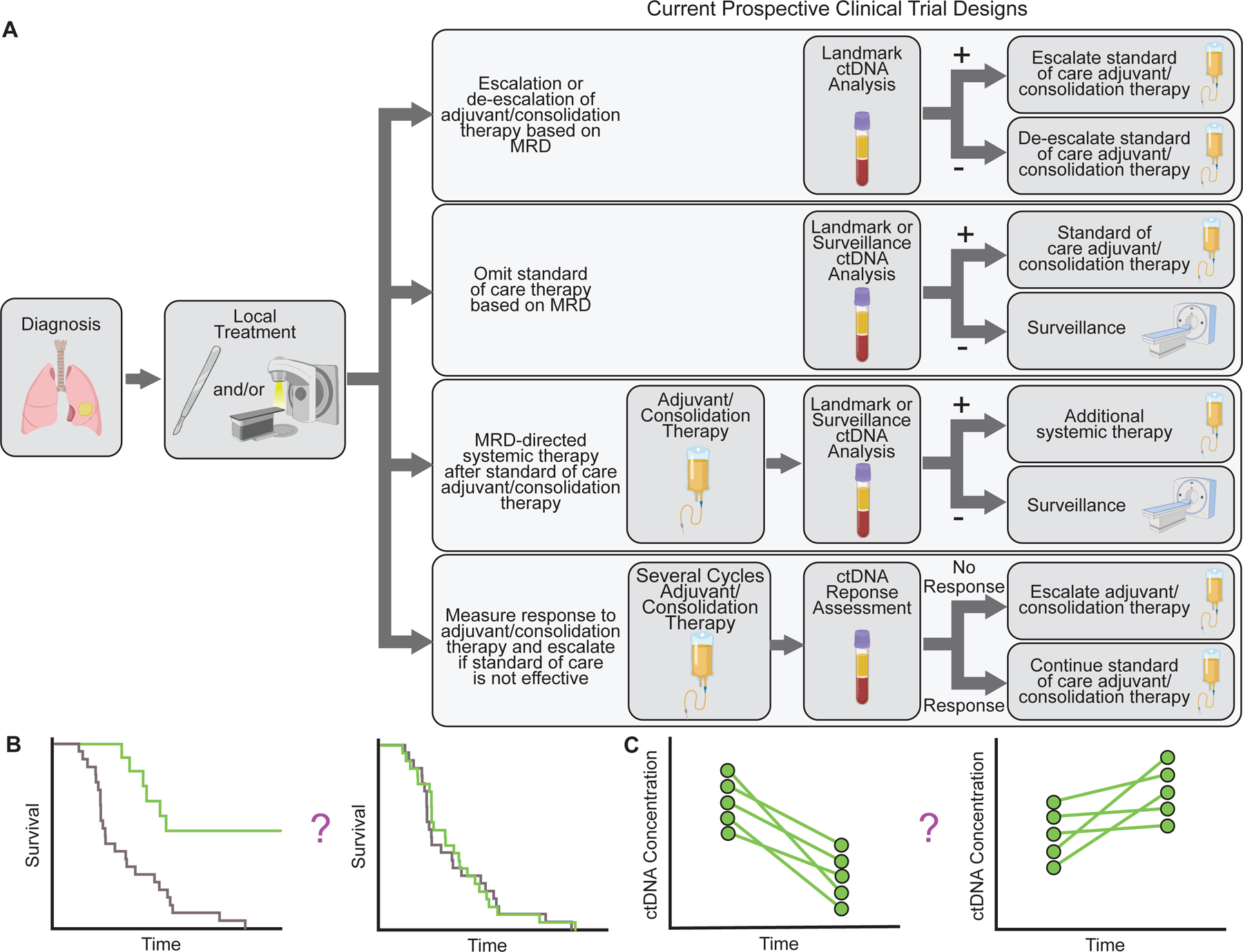
Examples of designs of ongoing interventional clinical trials testing personalization of adjuvant/consolidation therapy based on ctDNA MRD. A) Schematic depicting examples of ongoing interventional clinical trials using ctDNA MRD to guide treatment. B-C) Potential clinical trial endpoints for ctDNA MRD studies. B) Survival (overall survival, disease free survival, or event free survival) for patients managed based on ctDNA MRD can be compared with a control arm or historical cohort or patients managed according to standard of care. C) For patients with ctDNA MRD, ctDNA clearance or change in ctDNA concentration could be used as a surrogate endpoint.
