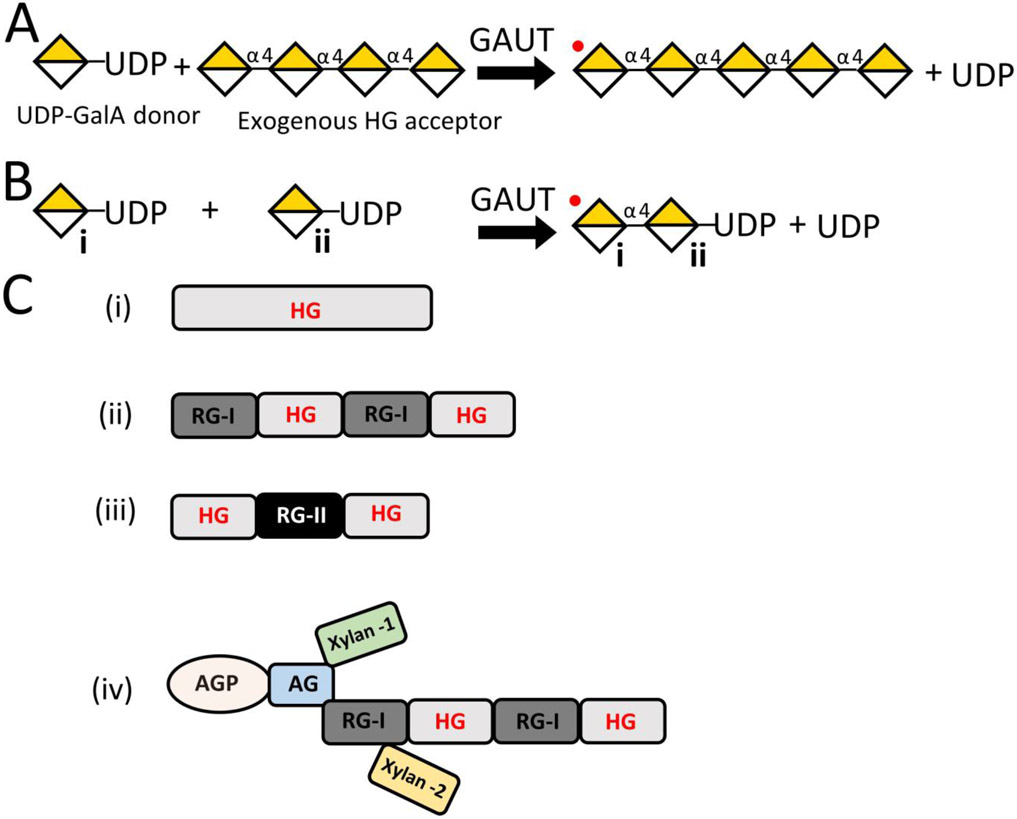
Figure 1. Schematic representation of homogalacturonan (HG) biosynthesis mechanisms and HG polymers that are synthesized in vitro or recovered from plant sources.
(A) Depiction of homogalacturonan galacturonosyltransferase (HG:GalAT) activity in the presence of UDP-GalA and exogenous HG acceptor. Multiple GAUTs are able to elongate exogenous HG acceptors (in this example, an acceptor length with a degree of polymerization of four) at the non-reducing end with GalA residues (yellow and white diamond) in the presence of UDP-GalA nucleotide sugar donor (Sterling et al., 2006; Atmodjo et al., 2011; Amos et al., 2018; Biswal et al., 2018a; Voinicuic et al., 2018). An HG homoglycan is generated that can be further glycosylated by addition of GalA to the non-reducing end (denoted by a red dot). (B) Depiction of HG:GalAT activity in the presence of UDP-GalA only. When no HG acceptor is accessible, data reported here show that at least some GAUTs can use UDP-GalA as an acceptor to synthesize and elongate HG, resulting in a polymeric HG product with UDP on its reducing end. The non-reducing end (denoted by a red dot) is available for subsequent GalA addition. This de novo synthesis activity has previously been detected for the GAUT1:GAUT7 complex but the presence of UDP on the reducing end could not be verified (Amos et al., 2018). In this paper we show that UDP is retained on the reducing end in product synthesized by GAUT13. (C) Depiction of HG or HG-containing structures either synthesized in vitro or recovered from plant cell walls. (i) Polymeric HG with a degree of polymerization > 500 can be synthesized in vitro (Amos et al., 2018), yet its existence in the cell wall as an exclusive polysaccharide, with no covalent linkages to other pectins, remains uncertain. HG isolated from cell walls has been shown to exist covalently connected via its backbone to (ii) RG-I, (iii) RG-II or (iv) as a component of the proteoglycan, APAP1 (Ishii & Matsunaga, 2001; Nakamura et al., 2002; Coenen et al., 2007; Tan et al., 2013).