Fig. 4: Enhanced T cell-mediated antitumor activity with Type I PRMT inhibitor treatment.
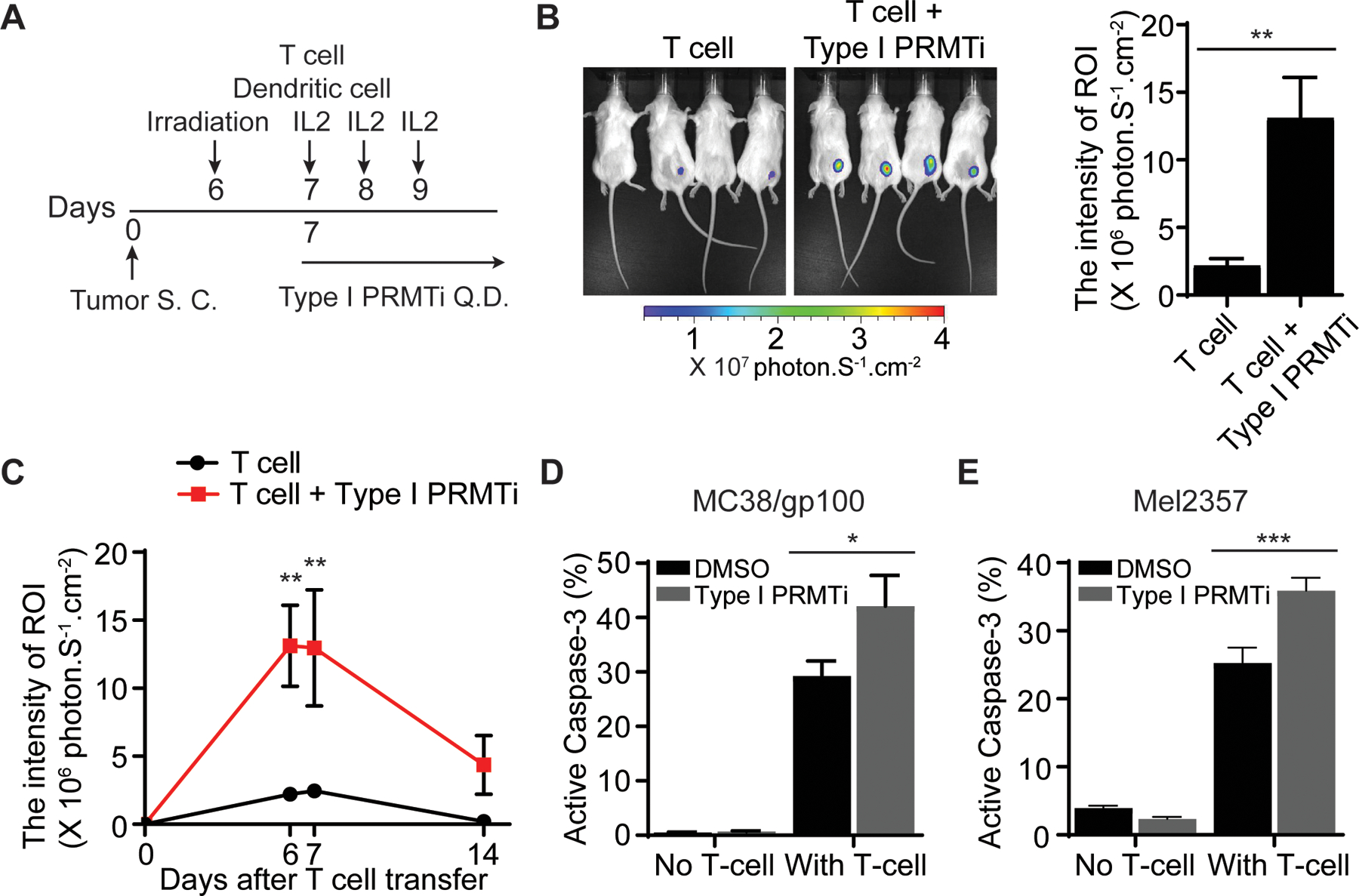
A, Schematic of the adoptive transfer model to evaluate the trafficking of tumor-reactive T cells in tumors. Luciferase-expressing pmel-1 T cells (1×106) were transferred into C57BL/6 albino mice bearing MC38/gp100 tumors (N=4–5). A DC vaccine and IL-2 treatment were administered. Either vehicle or Type I PRMTi (75 mg/kg/day) was orally administrated after T-cell transfer until the end of experiment. Luciferase intensity at the tumor sites was used to determine the trafficking of transferred tumor-reactive T cells. B, Representative images and quantitative imaging analysis showing the luciferase intensity of transferred T cells at tumor sites 6 days after T-cell transfer. Quantification was performed as the average of photon flux within region of interest (ROI). C, Time course of quantitative imaging analysis of tumor trafficking of transferred T cells. D-E, Type I PRMTi treatment increased sensitivity to T cell-mediated killing in vitro in (D) mouse MC38/gp100 tumor cells and (E) patient-derived melanoma Mel2357 cells. MC38/gp100 and Mel2357 tumor cells were pretreated with 0.1% DMSO or 2 μM Type I PRMTi for 6 days, re-seeded in fresh culture medium, followed by co-culture with pmel-1 T cells or autologous T cells (TIL2357), respectively. Tumor cell apoptosis was determined by the percentage of cleaved caspase-3+ tumor cells (N=3). All data are presented as means±SEM. Statistical significance was determined by student’s 2-tailed t-test (for panels B, D and E) or two-way repeated measures ANOVA (for panel C). *p≤0.05, **p≤0.01; ***p≤0.001.
