Figure 1.
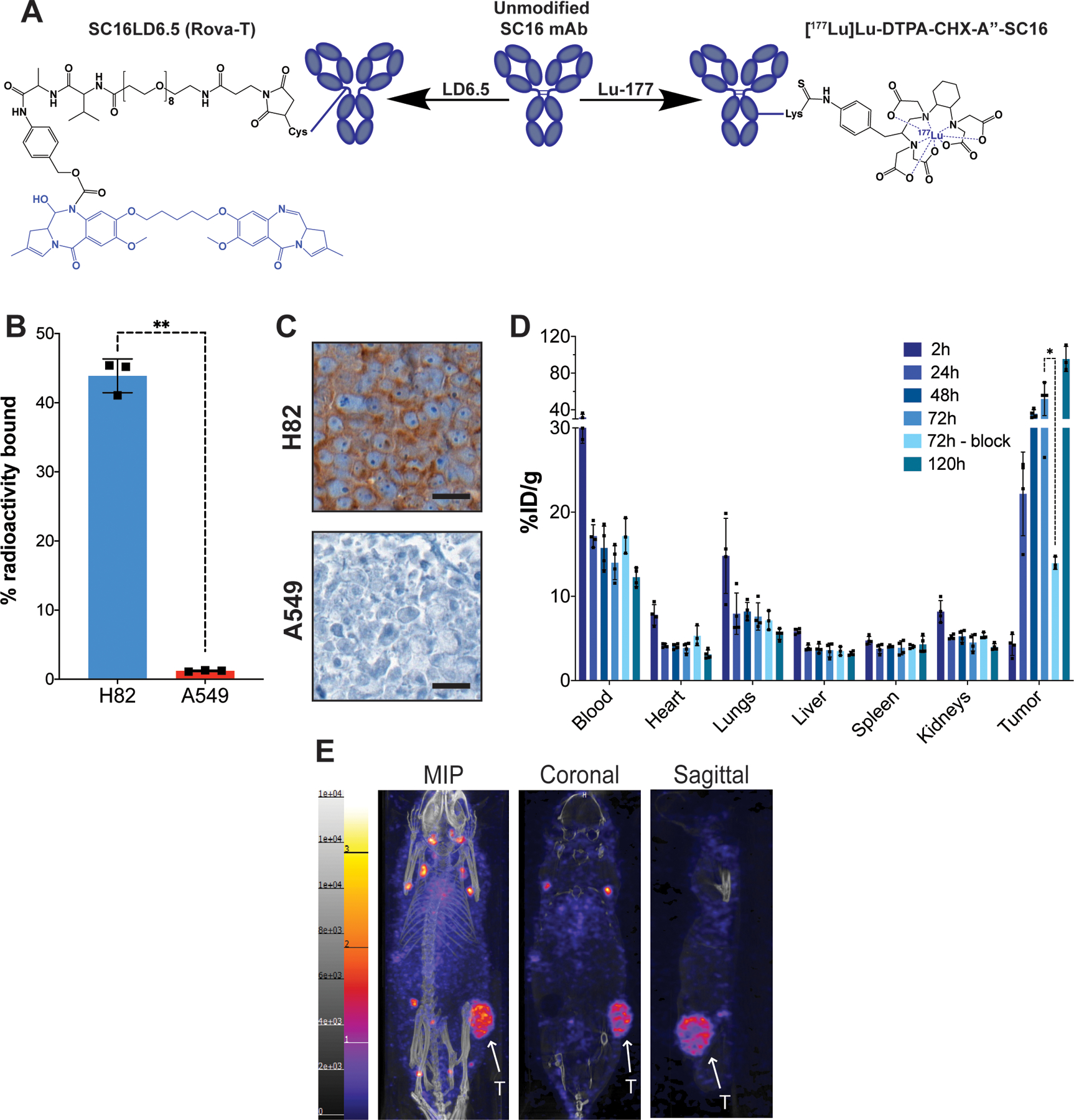
A) Representative structures of the unmodified SC16 antibody, the antibody-drug conjugate SC16LD6.5 (Rovalpituzumab tesirine), and the radioimmunoconjugate [177Lu]Lu-DTPA-CHX-A”-SC16. The cytotoxic moieties of each therapeutic are highlighted in blue. B) A radioligand binding assay of [177Lu]Lu-DTPA-CHX-A”-SC16 in H82 and A549 cells. Error bars represent SD (n=3). An unpaired, two-tailed t-test was performed (p-value = 0.001) C) DLL3 immunohistochemistry on subcutaneous tumor xenografts. Scale bar: 20 μm. See Figure S4 Mouse 108 for the lower magnification image of the H82 tumor DLL3 IHC and corresponding histology. D) The ex vivo biodistribution data of select organs from athymic mice bearing subcutaneous H82 xenografts after the administration of [177Lu]Lu-DTPA-CHX-A”-SC16 (10–30 μCi; 3 μg in 150 uL PBS; n=3 mice per time point) via tail vein. The tumor uptake at 72h was sufficiently blocked using 100-fold excess of unlabeled SC16 (p-value = 0.0212). See Figure S9 for the uptake in all organs collected. E) Representative whole body SPECT/CT images of athymic mice bearing subcutaneous H82 xenografts 120h after the administration of [177Lu]Lu-DTPA-CHX-A”-SC16 (1050–1100 μCi; 60–70 μg in 150 uL PBS) via tail vein. The tumors (indicated by white arrows) can clearly be delineated in the maximum intensity projection (MIP), coronal, and sagittal images.
