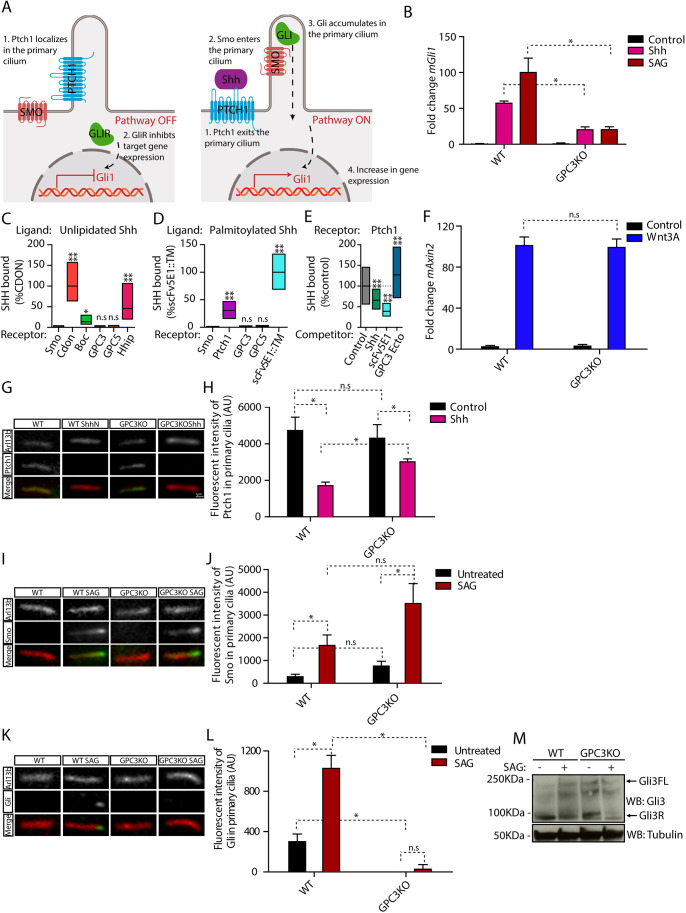
Fig. 1.
Endogenous GPC3 is necessary for Hh pathway activation. (A) Schematic of the Hh pathway. Left: in the absence of signaling, Ptch1 in primary cilia inhibits Smo activation and localization to cilia. Right: upon pathway activation by Shh, Ptch1 is inhibited and exits from cilia, whereas Smo becomes active and accumulates in cilia. Smo activates Gli proteins, which accumulate at ciliary tips. Active Gli proteins turn on the transcriptional targets of the pathway, including Gli1. (B) Wild-type (WT) or Gpc3KO MEFs were incubated with Shh, SAG (1 µM) or control medium for 24 h, and Hh signaling was measured by qRT-PCR for Gli1. Data are mean±s.e.m. of three replicates and were normalized from 0% (untreated) to 100% activation of the Hh pathway by saturating SAG. Hh pathway activation by Shh and SAG was significantly decreased in the absence of GPC3. (C) Fluorescently labeled unlipidated Shh was incubated with HEK293T cells transiently transfected with eGFP-tagged GPC3, GPC5 or control receptors, and bound ligand was quantified by fluorescence microscopy. Cdon, Boc and Hhip (left), as well as Ptch1 and a single-chain variant of the anti-Shh monoclonal antibody (scFv5E1) (Wierbowski et al., 2020) (right), were used as positive controls, and Smo was used as negative control. Data are normalized between binding to the negative control (0%) and the highest bound signal (100%). Boxplots represent the median and the first and third quartiles of binding. At least 200 cells were measured per condition. Neither GPC3 nor GPC5 binds unlipidated Shh. (D) As in C but using fluorescently labeled palmitoylated Shh. At least 1000 cells were measured per condition. Neither GPC3 nor GPC5 binds palmitoylated Shh. (E) As in C, except binding of palmitoylated Shh to Ptch1 was measured after preincubation of Shh with purified GPC3 ectodomain (GPC3-Ecto) or control competitors. Data are normalized between binding to Smo (negative control, 0%) and binding to Ptch1 in the presence of control competitor (100%). At least 1000 cells were measured per condition. GPC3-Ecto does not compete with Ptch1 for Shh binding. Shh binding was competed by scFv5E1 or by excess unlipidated Shh. (F) Wild-type or Gpc3KO MEFs were incubated with Wnt3A-conditioned medium or control medium for 24 h, and Wnt signaling was measured by qRT-PCR for Axin2. Wnt pathway activation by Wnt3A was normal in the absence of GPC3. Data are mean±s.e.m. of three replicates. (G) Wild-type or Gpc3KO MEFs stably expressing Ptch1-eGFP were incubated with Shh as in B, and ciliary intensity of Ptch1 was measured by immunofluorescence microscopy. Cilia were detected by staining for endogenous Arl13B. Ptch1 is green, Arl13B is red. In both wild-type and Gpc3KO cells, Shh induces Ptch1 exit from cilia. (H) Quantification of the experiment in G. Data are mean±s.d. (300-400 cilia were measured per condition). (I) As in G but measuring ciliary levels of endogenous Smo following treatment with SAG (1 µM). Smo is green, Arl13B is red. In both wild-type and Gpc3KO cells, SAG causes normal ciliary accumulation of Smo. (J) As in H but quantifying the experiment in I (300-400 cilia were measured per condition). (K) As in G but measuring ciliary intensity of endogenous Gli proteins. The anti-Gli antibody recognizes both full-length Gli2 and Gli3 (Tukachinsky et al., 2010). Gli is green, Arl13B is red. In the absence of GPC3, Gli proteins did not localize to the tips of cilia and their ciliary recruitment in response to SAG was greatly reduced. (L) As in H but quantifying the experiment in K (300-400 cilia were measured per condition). (M) As in B but cells were analyzed by immunoblotting for Gli3. Blotting for tubulin served as loading control. In the absence of GPC3, Gli3R was cleared normally by Hh pathway stimulation. Blots shown are representative of three experiments. *P<0.05; ****P<0.0001; n.s., not significant (two-tailed paired t-test). AU, arbitrary units. Scale bar: 1 μm (shown in G, also applies to I and K).