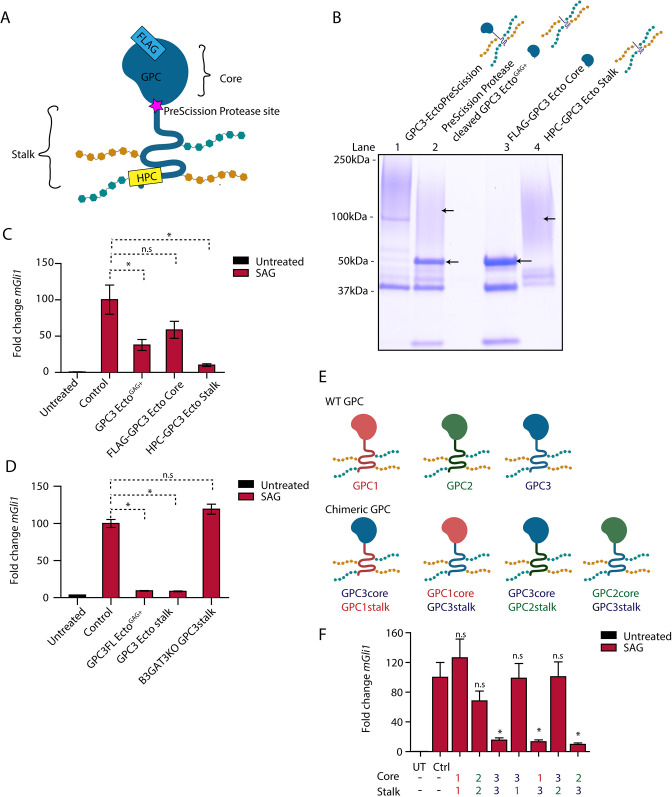
Fig. 5.
The GAG-modified GPC3 stalk is necessary and sufficient for Hh pathway antagonism. (A) Schematic of the GPC3-Ecto variant with an engineered PreScission protease cleavage site between the core and stalk domains (GPC3-EctoPreScission). The fusion is FLAG tagged on the N terminus (to isolate the core domain after cleavage, FLAG-GPC3core) and HPC tagged on the C terminus (to isolate the stalk region, HPC-GPC3 stalk). (B) Affinity-purified GPC3-EctoPreScission (lane 1) was cleaved with PreScission protease (lane 2), followed by purification of the core domain (lane 3) and stalk (lane 4) by FLAG and HPC affinity, respectively. Proteins were analyzed by SDS-PAGE and Coomassie staining. Following protease cleavage, the globular core collapsed to defined bands (lane 3), whereas the stalk region migrated as a high molecular weight smear (lane 4) due to GAG modification. Gel shown is representative of three experiments. (C) Wild-type (WT) MEFs were incubated with SAG (1 µM) in the absence or presence of the indicated purified proteins (1 µM) for 24 h. Hh signaling was measured by qRT-PCR for Gli1. The GAG-modified GPC3 stalk potently inhibited Hh signaling, in contrast to the unmodified globular core. Untreated is no treatment. Cells were kept in DMEM. (D) As in C but with incubation with GPC3 stalk expressed and purified from B3GAT3KO cells. The unmodified GPC3 stalk did not inhibit Hh signaling. (E) Schematic of GPCs GPC1, GPC2 and GPC3, and the chimeric GPCs generated by domain swapping. (F) As in C but with incubation with purified chimeric GPC ectodomains. Hh signaling antagonism required the presence of the GPC3 stalk.; Ctrl, cells treated with the buffer for the purified protein; UT, untreated. Data are mean±s.e.m. of three replicates. *P<0.05; n.s., not significant (two-tailed paired t-test).