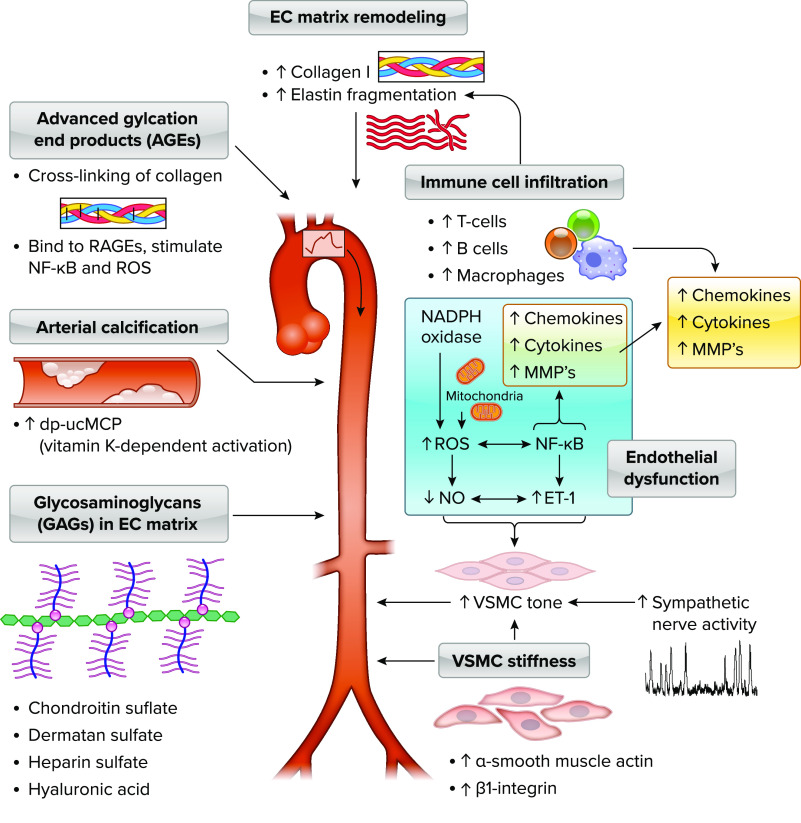
FIGURE 2.
Mechanisms and modulators of aortic stiffness The cellular mechanisms and modulators that influence aortic stiffness can be classified into alterations in the extracellular (EC) matrix in the arterial wall, modulators of arterial tone, intrinsic vascular smooth muscle cell (VSMC) stiffness, arterial calcification, and glycosaminoglycans (GAGs). Alterations in arterial tone can occur acutely, whereas the other mechanisms are typically chronic changes that can take months or years to occur but may be influenced by a variety of lifestyle or physiological stressors or pathologies. Extracellular matrix remodeling includes accumulation and reorganization of fibrotic proteins such as collagen I and III and degradation and fragmentation of elastin. Advanced glycation end products (AGEs), proteins or lipids that are formed from nonenzymatic glycation or oxidation after exposure to aldose sugars, accumulate in the vessel wall, leading to cross-links in extracellular matrix proteins with slow turnover rate such as collagen I and promotion of arterial stiffening. AGEs also bind to several different receptors for AGEs (RAGEs), which results in upregulation of the proinflammatory transcription factor nuclear factor-κB (NF-κB) and prooxidant genes leading to reactive oxygen species (ROS) production. Calcification of the aortic wall occurs in both the media and intima, and activation of unphospho-decarboxylated matrix Gla-protein (dp-ucMGP), a protein secreted by VSMCs and chondrocytes, is a vitamin K-dependent process, so that elevated levels of circulating inactive dp-ucMGP are associated with higher aortic stiffness. Extracellular matrix of elastin sheets and collagen fiber proteins, the medial layer of the aorta also contains GAGs, including chondroitin sulfate, followed by dermatan sulfate, heparin sulfate, and hyaluronan, that play some role in modulating elastic properties of the arterial wall. α-Smooth muscle actin, the most abundant isoform of actin in VSMCs, and β1-integrin, a key adhesion molecule that couples vascular smooth muscle and extracellular matrix, mediate a significant component of age-related increase in VSMC stiffness. Endothelial dysfunction, through elevated ROS and NF-κB-mediated reductions in nitric oxide (NO) and increased endothelin 1 (ET-1) and increased sympathetic nerve activity, converges with vascular smooth muscle stiffness to raise vascular smooth muscle tone. ROS and cytokines/chemokines such as transforming growth factor-beta (TGF-β) and matrix metalloproteinases (MMPs) work synergistically to alter extracellular matrix and fibrosis in the development of arterial stiffness. Advancing age is associated with increases in T and B cells and macrophages in the perivascular adipose tissue of the aorta in mice, and T and B cell knockout mice (RAG−/−) have a lower rate of age-related aortic stiffening across the life span.