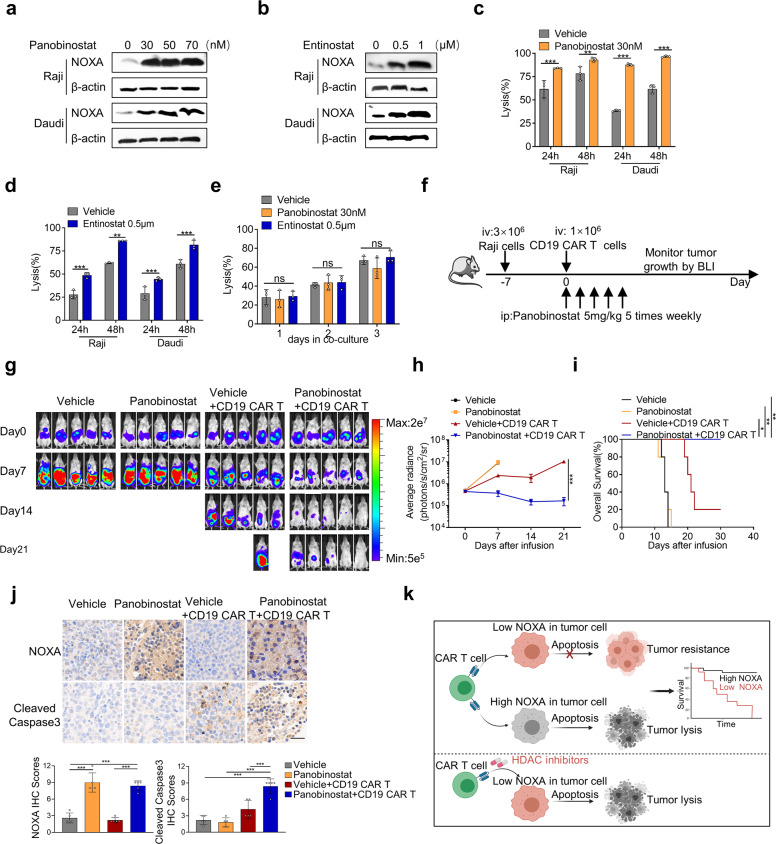
Fig. 6.
Histone deacetylase inhibitors could enhance tumor cell vulnerability to CAR T cells. a, b Western blot analysis of Raji cells and Daudi cells treated with panobinostat (a) or entinostat (b) for 24 h. c, d Cytotoxic analysis of Raji cells and Daudi cells pretreated with 30 nM panobinostat (c) or 0.5 μM entinostat (d) for 24 h and then cocultured with CD19 CAR T cells over time. e Cytotoxic analysis of NOXAKO Nalm6 cells pretreated with 30 nM panobinostat or 0.5 μM entinostat for 24 h and then cocultured with CD19 CAR T cells over time. Values in (a–e) were shown as the mean ± SD of triplicates. f Experimental timeline comparing the antitumor ability of vehicle alone, panobinostat alone, the combination of vehicle and CD19 CAR T cells, and combination of panobinostat and CD19 CAR T cells in mice bearing Raji-luc xenograft tumors (n = 5 mice per group). g Raji tumor progression as evaluated by bioluminescence imaging. h Mouse tumor burden (average radiance). The indicated P value was determined by two-way ANOVA test. i Survival analyses of mice treated with vehicle alone, panobinostat alone, the combination of vehicle and CD19 CAR T cells, and combination of panobinostat and CD19 CAR T cells. Log-rank tests were used to analyze the significance between four groups. j Representative images of IHC staining for NOXA and cleaved caspase3 from Raji tumor xenografts treated with the indicated treatments. Magnification: ×40, Scale bars: 20 μm. Quantification of IHC staining using IHC scores was represented as mean ± SD of five mice per group. P values were calculated with the two-tailed Student’s t test. k Schematic drawing summarizing our findings. Values are shown as the mean ± SD. ns: not significant (P > 0.05); *P < 0.05, **P < 0.01, and ***P < 0.001