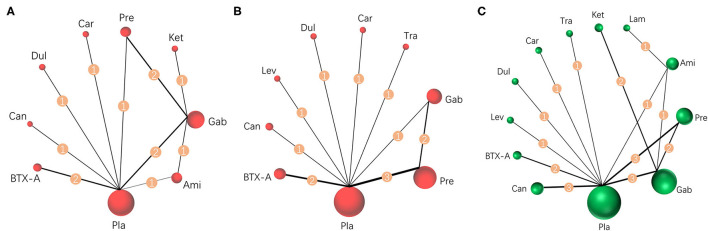
Figure 3.
Network meta-analysis of eligible comparisons for secondary outcomes. (A) Pain relief more than 8 weeks, n = 615. (B) Mental or sleep-related symptom relief, n = 825. (C) Serious adverse events, n = 1,232. Width of the lines is proportional to the number of trials, comparing every pair of treatments for secondary outcomes (numbers on the lines). Size of every circle is proportional to the number of randomly assigned participants (sample size). BTX-A, botulinum toxin-A; Ket, ketamine; Ami, amitriptyline; Lam, lamotrigine; Pre, pregabalin; Dul, duloxetine; Gab, gabapentin; Tra, tramadol; Lev, levetiracetam; Car, carbamazepine; Can, cannabinoids; Pla, placebo.