Abstract
Lung transplant recipients have an increased risk for severe coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A third dose of a SARS-CoV-2 vaccine has been recommended for all solid organ transplant recipients, but data from lung transplant recipients specifically are scarce. In this study, the serologic response to a third dose of an mRNA-based SARS-CoV-2 vaccine was measured in 78 lung transplant recipients. Sixty-two percent (n = 48) had a serological response to vaccination, which was significantly higher than after the second vaccine dose (27 patients (35%); p = 0.0013). A positive serologic response was associated with having had COVID-19 (p = 0.01), and higher serum IgG level and complement mannose binding lectin pathway activity prior to vaccination (p = 0.04 and p = 0.03, respectively). Serologic response was not associated with the dose of mycophenolate mofetil or prednisone or other immune status parameters. Eleven patients (14%) developed COVID-19 after the second or third vaccine dose, but this did not associate with serologic response after the second vaccine dose (9% in patients who developed COVID-19 versus 39% in patients who did not develop COVID-19 (p = 0.09)), or with serologic response above cut-off values associated with clinical protection in previous studies. In conclusion, the response to mRNA-based SARS-CoV-2 vaccines in lung transplant recipients improves significantly after a third vaccine dose. Factors associated with a positive serologic response are having had COVID-19 prior to vaccination, and serum IgG and complement mannose binding lectin pathway activity prior to vaccination. Serologic response did not associate with clinical protection against COVID-19 in this study.
Keywords: Lung transplant, SARS-CoV-2, COVID-19, Vaccination response
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. Introduction
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is recommended for all solid organ transplant recipients, in order to protect them against severe coronavirus disease 2019 (COVID-19). For lung transplant recipients, impaired response to vaccination might be an especially pressing issue, as they usually receive more intensive immunosuppressive therapy compared to other solid organ transplant recipients. Indeed, the observed serologic response in lung transplant recipients after two doses of an mRNA-based SARS-CoV-2 vaccine is poor [1]. It is recommended to give a third vaccine dose to all solid organ transplant recipients [2], but there are few reports on the serologic response to a third vaccine dose in lung transplant recipients specifically [[3], [4], [5]]. We previously reported on the serologic response to two doses of an mRNA-based SARS-CoV-2 vaccine in 91 lung transplant recipients [1]. We now analyzed the serologic response to a third dose of an mRNA-based SARS-CoV-2 vaccine in the same cohort.
2. Methods
Participants were included from the total cohort of lung transplant recipients that were followed up at St. Antonius Hospital in Nieuwegein, the Netherlands as of March 2021. This hospital is a referral center for lung transplantation in collaboration with University Medical Center Utrecht, the Netherlands. The patient population comprises all types of end-stage lung disease, except for cystic fibrosis. Standard immunosuppressive therapy used after lung transplantation consisted of tacrolimus, mycophenolate mofetil and prednisone. Doses are higher directly after transplantation, but after the first year post-transplantation, regular doses were based on blood levels (target level 7–10 μg/mL) for tacrolimus, and were 500 mg twice daily for mycophenolate mofetil and 10 mg once daily for prednisone.
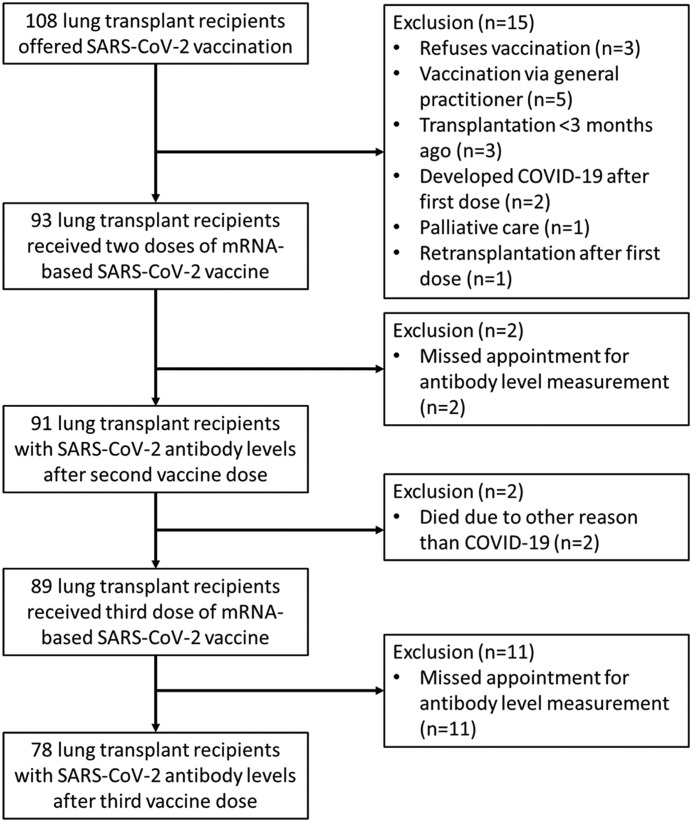
The study flow chart is shown in Fig. 1 . In total 108 patients were offered vaccination with two doses of an mRNA-based SARS-CoV-2 vaccine. The vaccines included BNT162b2 or mRNA-1273 [6,7], depending on what priority group patients were in. In accordance with national guidelines BNT162b2 was offered to healthcare personnel and people with other high-risk professions, and mRNA-1273 was offered to persons at an increased risk for severe COVID-19, including lung transplant recipients. The former group had a higher priority ranking, and therefore some lung transplant recipients employed in healthcare were vaccinated earlier. The initial vaccinations were scheduled in April and May 2021. The second dose was given approximately four weeks after the first dose. SARS-CoV-2 IgG levels were measured prior to the first vaccination, then four weeks after the first vaccination (prior to the second vaccination), and ten weeks after the first vaccination (i.e. six weeks after the second vaccination). The third dose was scheduled from November 2021 onward, and in most cases BNT162b2 was used as this was nationally allocated for persons at an increased risk for severe COVID-19. Follow up was completed up to January 2022.
Fig. 1.
Study flow chart.
SARS-CoV-2 spike S1/S2 protein specific IgG antibody levels were measured on the Liaison platform (DiaSorin, Saluggia, Italy). A positive serologic response was defined as having detectable IgG antibodies against SARS-CoV-2 after the second or third vaccination (cut-off >33.8 BAU/ml). The lower and upper detection limits of the assay were 4.81 BAU/ml and 2080 BAU/ml, respectively. Previous studies have suggested that antibody levels >94 BAU/mL after two BNT162b2 vaccine doses associated with 67% of individuals being protected against infection with the delta variant [8], and antibody levels >300 BAU/mL after two mRNA-1273 doses associated with 90% vaccine efficacy. We have evaluated the vaccine response in this cohort in relation to those tresholds [9].
Clinical characteristics and immune status parameters were retrieved from patient records. Immunoglobulin levels and IgG-subclasses, as well as antibodies against pneumococcal polysaccharides are routinely followed up. Only measurements that were taken less than six months prior to receiving the first dose of the SARS-CoV-2 vaccine were included. Response to pneumococcal vaccination, as well as antibodies to protein antigens, and complement level measurements were done prior to transplantation. Pneumococcal vaccinations were repeated post-transplantation, usually five years after the pre-transplantation pneumococcal vaccination.
The pneumococcal vaccination schedules and methods for measuring pneumococcal antibody levels have been previously described [10]. The 2015 AAAAI/ACAAI classification was used for overall categorization of the antibody response to pneumococcal vaccination [11].
SPSS Statistics for Windows (version 26.0; Armonk, NY) was used for data collection, statistical analyses, and designing of graphs. For comparison between two groups, the students t-test, Fisher exact test, Mann-Whitney U test and Wilcoxon signed ranks test were used where appropriate. For the multivariate model, logistic regression analysis was used. A p-value of <0.05 was considered to be statistically significant. All patients gave written informed consent for the use of their data in clinical research, and this was approved by the local ethics committee. The study was conducted in accordance with the Declaration of Helsinki.
3. Results
Eighty-nine patients received a third vaccine dose (BNT162b2 (n = 85) or mRNA-1273 (n = 4)) a median of 182 days after the second dose (interquartile range 175–198). Two patients had died before a third vaccine dose was offered, due to causes unrelated to COVID-19. Antibody levels were measured in 78 patients (Table 1 ). Thirty-eight patients were female (49%) and the median age at the time of the first vaccination was 62 years (interquartile range 53–67 years). The median time since lung transplantation was 4.46 years (interquartile range 1.84–8.14) and 69 patients had received a double lung transplantation (89%). Seventy patients received standard immunosuppressive therapy at the time of vaccination (90%) and fifteen patients were receiving antibody replacement therapy (19%). Thirteen patients had had COVID-19 prior to their first vaccination (17%).
Table 1.
Baseline clinical characteristics for 78 lung transplant recipients who received three doses of mRNA-based SARS-CoV-2 vaccine and for whom antibodies against SARS-CoV-2 were measured.
| Female (%) | 38 (49) |
|---|---|
| Median age in years (interquartile range) | 62 (53–67) |
| Median years since transplantation (interquartile range) | 4.46 (1.84–8.14) |
| Double lung transplantation (%) | 69 (89) |
| Diagnosis prior to transplantation (%) | |
|
27 (35) |
|
45 (58) |
|
1 (1) |
|
2 (3) |
|
3 (4) |
| Immunosuppressive therapy (%) | |
|
70 (90) |
|
5 (6) |
|
1 (1) |
|
2 (3) |
| Receiving antibody replacement therapy (%) | 15 (19) |
| Documented COVID-19 prior to vaccination (%) | 13 (17) |
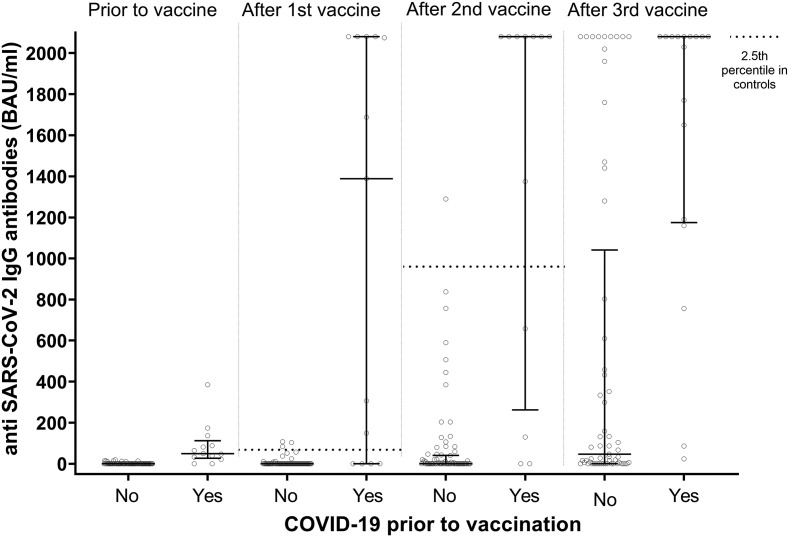
After the third vaccine dose, 48 patients (62%) had a serological response to vaccination, which is significantly higher than after the second vaccine dose (27 patients (35%); p = 0.0013) (Fig. 2 ). The median antibody concentration after the second vaccine dose was 0.00 BAU/mL (interquartile range 0.00–111.80). The median antibody concentration after the third vaccine dose was 132.50 BAU/mL (interquartile range 5.90–2022.50). In contrast to the serologic response after two vaccine doses, response after the third dose was not associated with a lower dose of prednisone (p = 0.39) or mycophenolate mofetil (p = 0.08), or younger age at vaccination (p = 0.09) (Table 2 ). A serologic vaccine response was associated with having had COVID-19 prior to vaccination (p = 0.01). When comparing the vaccine response in lung transplant recipients to previously suggested thresholds for clinical protection against COVID-19, 41 patients (53%) had antibody levels >94 BAU/mL, and 36 patients (46%) had antibody levels >300 BAU/mL.
Fig. 2.
Grouped scatter plot showing anti-SARS-CoV-2 IgG antibodies in lung transplant recipients prior to vaccination with SARS-CoV-2 vaccine, four weeks after the first vaccine dose, six weeks after the second vaccine dose, and a median of 55 days after the third vaccine dose (interquartile range 42–75) after the third vaccine dose. Patients were only categorized as having had COVID-19 when they had had COVID-19 prior to antibody level measurement. Error bars represent the group median with 25th and 75th percentiles. All values below the lower detection limit of the assay were set to 0. After the third vaccine, antibody levels were significantly higher in patients who had had COVID-19 prior to antibody level measurement (n = 17) compared to patients who had not had COVID-19 (n = 61; median 2080 BAU/ml (IQR 1175–2080) versus 47 (0–1042); p < 0.001). In all but two patients, the antibody level after the third vaccine dose was higher than the antibody level after the second vaccine dose. The dashed line represents the 2.5th percentile value of anti-SARS-CoV-2 IgG antibodies after the first and second vaccination in 102 healthy volunteers, as provided by the manufacturer (after the first vaccination the 2.5th percentile was 64.9 BAU/ml, and the median value 358.8 BAU/ml; after the second vaccination the 2.5th percentile was 966.1 BAU/ml, and the median value 4435.6 BAU/ml). There are no reference values available for the response after the third dose in healthy volunteers.
Table 2.
Antibody response to three doses of mRNA-based SARS-CoV-2 vaccine in 78 lung transplant recipients.
| Positive response (n = 48) | Negative response (n = 30) | p-value | |
|---|---|---|---|
| Female (%) | 23 (48) | 15 (50) | 0.86 |
| Median age (IQR) | 61 (50–65) | 63 (55–68) | 0.09 |
| Median years since transplantation (IQR) | 4.28 (1.87–8.06) | 5.36 (1.62–10.52) | 0.85 |
| Non-standard immunosuppressive therapy (%) | 4 (8) | 4 (13) | 0.48 |
| Tacrolimus (%) | 47 (98) | 30 (100) | 1.00 |
| Sirolimus added to tacrolimus (%) | 2 (4) | 0 (0) | 0.52 |
| Mycophenolate mofetil (%) | 47 (98) | 26 (87) | 0.08 |
|
7 (15) | 4 (13) | |
|
37 (77) | 17 (57) | |
|
2 (7) | 6 (10) | |
| Prednisone (%) | 48 (100) | 30 (100) | 0.39 |
|
11 (23) | 4 (13) | |
|
35 (73) | 23 (77) | |
|
2 (4) | 3 (10) | |
| COVID-19 prior to vaccination (%) | 12 (25) | 1 (3) | 0.01 |
| Positive SARS-CoV-2 antibodies prior to first vaccination (%) * | 9 (19) | 1 (3) | 0.08 |
| Positive SARS-CoV-2 antibodies prior to second vaccination (%) # | 15 (31) | 1 (3) | 0.01 |
| Positive SARS-CoV-2 antibodies after second vaccination (%) | 25 (52) | 2 (7) | <0.001 |
IQR = interquartile range. Percentages for subcategories are based on the number of patients for whom that parameter was available. * available in 77 patients. # available in 76 patients. Positive response defined as an IgG concentration > 33.8 BAU/ml.
Results for immune status investigations are shown in Table 3 . Total serum IgG levels were higher in patients who had a serologic response to vaccination (6.81 (interquartile range 6.12–8.39) versus 7.20 (6.46–8.04); p = 0.04) The median complement mannose binding lectin pathway activity was significantly higher in patients who had a serologic response to vaccination (98.5 (interquartile range 75.8–118.3) versus 68 (26–111.5); p = 0.03). Other serum immunoglobulin levels and IgG subclass levels, as well as response to pneumococcal vaccination prior to and after lung transplantation, were comparable between patients with a positive antibody response to the SARS-CoV-2 vaccine and patients with a negative response to the SARS-CoV-2 vaccine. Antibody replacement therapy was not associated with a response to the SARS-CoV-2 vaccine.
Table 3.
Immune status parameters in 78 lung transplant recipients who received three doses of mRNA-based SARS-CoV-2 vaccine.
| Positive response (n = 48) | Negative response (n = 30) | p-value | |
|---|---|---|---|
| Median IgM g/L (IQR) | 0.58 (0.47–0.84) | 0.65 (0.40–1.43) | 0.16 |
| Median IgA g/L (IQR) | 1.97 (1.06–2.42) | 2.45 (1.43–3.09) | 0.80 |
| Median IgG g/L (IQR) | 6.81 (6.12–8.39) | 7.20 (6.46–8.04) | 0.04 |
| Median IgG1 g/L (IQR) | 4.54 (3.85–6.26) | 5.08 (4.61–5.54) | 1.00 |
| Median IgG2 g/L (IQR) | 1.56 (1.26–2.11) | 1.44 (1.33–1.93) | 0.58 |
| Median IgG3 g/L (IQR) | 0.19 (0.15–0.28) | 0.24 (0.20–0.35) | 0.86 |
| Median IgG4 g/L (IQR) | 0.16 (0.11–0.32) | 0.22 (0.08–0.31) | 0.64 |
| Median pneumococcal serotypes >1.3 μg/mL (IQR) * | 3.5 (2–6) | 4 (2–6) | 0.89 |
| Response to pneumococcal vaccination prior to transplantation | 39 (81) | 29 (97) | 0.08 |
| Normal (%) | 26 (67) | 22 (76) | 0.44 |
| Moderately impaired (%) | 12 (31) | 6 (21) | 0.41 |
| Severely impaired (%) | 1 (3) | 1 (3) | 1.00 |
| Response to pneumococcal vaccination after transplantation | 25 (52) | 17 (57) | 0.87 |
|
8 (32) | 4 (24) | 0.73 |
|
13 (52) | 13 (76) | 0.19 |
|
4 (16) | 0 (0) | 0.13 |
| Median complement mannose binding lectin pathway activity (IQR) | 98.5 (75.8–118.3) | 68 (26–111.5) | 0.03 |
| Any respiratory tract infection in the year prior to vaccination (%) # | 11 (26) | 12 (48) | 0.17 |
| Antibody replacement therapy (%) | 8 (17) | 7 (23) | 0.47 |
Percentages for subcategories are based on the number of patients for whom that parameter was available. IQR = interquartile range; MP = mannose-binding lectin pathway. * available in 55 patients, measured <6 months prior to the first dose of a SARS-CoV-2 vaccine. # only available in 67 patients transplanted at least a year prior to vaccination with a SARS-CoV-2 vaccine (42 with a positive antibody response and 25 with a negative antibody response).
Eleven patients (14%) developed COVID-19 after the second vaccine dose. One patient developed COVID-19 between the second and third vaccine dose and ten patients developed COVID-19 after the third vaccine dose. Of these eleven patients, one had a serologic response after the second dose (9%), compared to 26 of 67 patients (39%) who did not develop COVID-19 (p = 0.09). One patient who developed COVID-19 after the third vaccine dose died, and all other patients recovered. Fifty-five patients did not have COVID-19 at any point, and antibody levels after the second vaccine dose were positive in 16 (29%) of these patients, compared to positive antibody levels in 27 of 55 patients (49%) after the third vaccine dose. Patients who did not develop COVID-19 after the third vaccine dose had median antibody levels of 109.85 BAU/mL (interquartile range 1.48–1767.50) after the third vaccine dose, compared to 1106.00 (85.03–2080.00) in patients who did develop COVID-19 after the third vaccine dose (p = 0.10). Antibody levels were > 94 BAU/mL after the third vaccine dose in 7 out of 10 patients who subsequently developed COVID-19 (p = 0.31 compared to patients who did not develop COVID-19 after the third vaccine dose). Antibody levels were > 300 BAU/mL after the third vaccine dose in 5 out of 10 patients who subsequently developed COVID-19 (p = 1.00 compared to patients who did not develop COVID-19 after the third vaccine dose).
4. Discussion
This study shows that 62% of lung transplant recipients have a serologic response after the third dose of a SARS-CoV-2 mRNA-based vaccine. This is significantly higher than a 35% serologic response rate after the second vaccine dose. Factors associated with a better serologic response were having had COVID-19 prior to vaccination, and having higher serum IgG or complement mannose binding lectin activity prior to vaccination. Serologic response to the SARS-CoV-2 vaccines was not associated with the dose of mycophenolate mofetil or prednisone or other immune status parameters.
These findings are in line with those from studies in other solid organ transplant recipient, where similar seroconversion rates after the third vaccine dose were observed [4]. Interestingly, neither serum IgG level nor complement mannose binding lectin activity associated with the serologic response after the second vaccine dose [1]. Hypogammaglobulinemia was found to be associated with a decreased response to mRNA-based SARS-CoV-2 vaccines in liver and heart transplant recipients in a previous study [12]. The relation of complement mannose binding lectin activity with the response to other vaccines has been investigated, but no clear relation has been found [13,14]. Yet, mannose-binding lectin recognition of the SARS-CoV-2 spike protein does seem to be relevant in the pathogenesis of COVID-19 [15].
Fourteen percent of the lung transplant patients in this cohort developed COVID-19 after the second or third vaccine dose. Importantly, this did not associate with the serologic response after the second vaccine dose, although median antibody levels after the third vaccine dose were nominally higher in patients who did not develop COVID-19. Notably, vaccination seems to offer a lower degree of protection against the SARS-CoV-2 Omicron variant [16,17]. However, the Delta variant was the dominant circulating variant in The Netherlands at the time that all but two patients who developed COVID-19 [18]. This indicates that lung transplant recipients are still at a high risk for developing COVID-19 despite vaccination. In this study only one patient died from COVID-19, but to what degree vaccination provides protection against severe COVID-19 in lung transplant patients cannot be definitively stated.
The present study has several limitations. First, we only focused on serologic response to vaccination, whereas protection against severe COVID-19 is a more important outcome measure. SARS-CoV-2 vaccines have been shown to protect solid organ transplant recipients against severe COVID-19 [19], but it remains to be determined to what degree serologic response correlates with clinical protection in solid organ transplant recipients. Even for the general population, clinically relevant cut-off values for antibody levels that associate with clinical protection are not yet available [20]. In some solid organ transplant recipients a cellular immune response to SARS-CoV-2 vaccination has been observed even in the absence of a serologic response [21]. However, routinely testing the cellular immune response or neutralizing antibodies is difficult in clinical practice. Measuring the serologic response, on the other hand, can be performed relatively easily. This might be used to decide which patients could benefit from booster vaccination doses. A second limitation of this study is that we were unable to include an appropriate control group. However, available data, generated with the same laboratory platform, indicate that almost all healthy individuals have a relevant and durable serologic response to SARS-CoV-2 vaccines [22]. Third, data on immune status parameters was collected retrospectively and was missing in some patients. Finally, as in most studies on lung transplant recipients, this study has a relatively small sample size. The lack of association between vaccine response and the dose of immunosuppressive medication or clinical protection against COVID-19 might be related to this small sample size.
In conclusion, the serologic response to mRNA-based SARS-CoV-2 vaccines in lung transplant recipients improved after a third vaccine dose. Having had COVID-19 prior to vaccination was associated with a better vaccine response, but in almost all cases antibody levels obtained after the 3rd vaccine dose were higher than after the 2nd vaccine dose. Multiple breakthrough infections were observed, but this did not associate with serologic vaccination response.
Disclosures
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
Acknowledgements
We thank Ms. M. Jansen-Hagen, Ms. M.B.F. Langezaal, Ms. H. Snijders-Tijink, and Ms. M.C. de Wit for their assistance with study planning and data collection.
Authorship
DAvK conceived of the study. TWH, BM and DAvK participated in research design. TWH collected data and performed the data analyses. TWH, BM, GTR, and DAvK participated in the interpretation of the data. TWH wrote the first draft of the manuscript. BM, GTR and DAvK critically reviewed and revised the manuscript.
References
- 1.Hoffman T.W., Meek B., Rijkers G.T., Van Kessel D.A. Poor serologic response to 2 doses of an mRNA-based SARS-CoV-2 vaccine in lung transplant recipients. Transplantation. 2022;106(1):E103–E104. doi: 10.1097/TP.0000000000003966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ISHLT, AST Joint Statement about Vaccine Efficacy in Organ Transplant Recipients. 2021. https://ishlt.org/ishlt/media/documents/ISHLT-AST_SARS-CoV-2-Vaccination_8-13-21.pdf
- 3.Efros O., Anteby R., Halfon M., Meisel E., Klang E., Soffer S. Efficacy and safety of third dose of the COVID-19 vaccine among solid organ transplant recipients: a systemic review and meta-analysis. Vaccines. 2022;10(1) doi: 10.3390/VACCINES10010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamar N., Abravanel F., Marion O., et al. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am. J. Transplant. 2022 doi: 10.1111/AJT.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havlin J., Skotnicova A., Dvorackova E., et al. Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein-specific t cells in lung transplant recipients. Transplantation. 2021 doi: 10.1097/TP.0000000000004021. Publish ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., et al. 383(27) 2020. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine; pp. 2603–2615. doi: 101056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L.R., El Sahly H.M., Essink B., et al. 384(5) 2020. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine; pp. 403–416. doi: 101056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J., Pouwels K.B., Stoesser N., et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022:1–11. doi: 10.1038/s41591-022-01721-6. February 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert P.B., Montefiori D.C., McDermott A.B., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science (80- ). 2022;375(6576):43–50. doi: 10.1126/SCIENCE.ABM3425/SUPPL_FILE/SCIENCE.ABM3425_STATISTICAL_ANALYSIS_PLAN.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman T.W., Meek B., Rijkers G.T., Grutters J.C., van Kessel D.A. Pneumococcal conjugate vaccination followed by pneumococcal polysaccharide vaccination in lung transplant candidates and recipients. Transplant. Direct. 2020;6(6) doi: 10.1097/TXD.0000000000001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonilla F.A., Khan D.A., Ballas Z.K., et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J. Allergy Clin. Immunol. 2014;136(5):1186–1205.e78. doi: 10.1016/j.jaci.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Herrera S., Colmenero J., Pascal M., et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am. J. Transplant. 2021;21(12):3971–3979. doi: 10.1111/AJT.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kessel D.A., Hoffman T.W., van Velzen-Blad H., Zanen P., Rijkers G.T., Grutters J.C. Response to pneumococcal vaccination in mannose-binding lectin-deficient adults with recurrent respiratory tract infections. Clin. Exp. Immunol. 2014;177(1):272–279. doi: 10.1111/cei.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gröndahl-Yli-Hannuksela K., Vuononvirta J., Peltola V., Mertsola J., He Q. Lack of association between mannose binding lectin and antibody responses after acellular pertussis vaccinations. PLoS One. 2014;9(2) doi: 10.1371/JOURNAL.PONE.0088919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stravalaci M., Pagani I., Paraboschi E.M., et al. Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules. Nat. Immunol. 2022;23(2):275–286. doi: 10.1038/s41590-021-01114-w. [DOI] [PubMed] [Google Scholar]
- 16.Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N. Engl. J. Med. 2022;386(5):494–496. doi: 10.1056/NEJMC2119270/SUPPL_FILE/NEJMC2119270_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accorsi E.K., Britton A., Fleming-Dutra K.E., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–651. doi: 10.1001/JAMA.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RIVM Surveillance of SARS-CoV-2 Variants in The Netherlands. 2022. https://www.rivm.nl/sites/default/files/2022-02/Kiemsurveillancetabel20220225NL.pdf Published. (Accessed February 26, 2022)
- 19.Aslam S., Liu J., Sigler R., et al. Coronavirus disease 2019 vaccination is protective of clinical disease in solid organ transplant recipients. Transpl. Infect. Dis. January 2022 doi: 10.1111/TID.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra A., Theel E.S. Immunity to SARS-CoV-2: what do we know and should we be testing for it? Humphries RM, ed. J. Clin. Microbiol. 2022 doi: 10.1128/JCM.00482-21. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havlin J., Svorcova M., Dvorackova E., et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transplant. 2021;0(0) doi: 10.1016/J.HEALUN.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widge A.T., Rouphael N.G., Jackson L.A., et al. 384(1) 2020. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination; pp. 80–82. doi: 101056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.