Abstract
A facile photoinduced successive oxidative ring-opening and borylation of indolizines with NHC–boranes via a one-pot method has been unveiled. This photo-promoted strategy enables the formation of unsaturated NHC–boryl carboxylates under transition metal-free and radical initiator-free conditions. A wide array of pyridine-containing NHC–boryl carboxylates were directly prepared in moderate to good yields. This work contributes to a better understanding of the reactivity and photo-behavior of both indolizines and NHC–boranes.
A facile photoinduced successive oxidative ring-opening and borylation of indolizines with NHC–boranes via a one-pot method has been unveiled. This photo-promoted strategy enables the formation of unsaturated NHC–boryl carboxylates under transition metal-free conditions.
Organoboron compounds are of vital significance in modern organic synthesis, and they also show great promise in luminescent materials, covalent organic frameworks, and pharmaceutical industries.1–3N-Heterocyclic carbene (NHC)–boranes have recently gathered a lot of attention in building various C–B bonds.4 In fact, as an increasingly popular and reliable boron source, NHC–boranes have the typical characteristics of being: (1) weaker B–H bonds compared to other LB·BH3; (2) stable solids under ambient conditions; (3) easily prepared on the gram scale. Owing to these advantages, many borylation methods via NHC–boryl radicals have been extensively exploited.5 Among them, photochemical borylations with high efficiency have become a powerful, practical, and economic strategy.6 For example, Xie and Zhu reported a photoinduced inverse hydroboration of imines and alkenes with NHC–boranes.7 Visible-light-induced selective defluoroborylation of fluorinated alkenes have been well established by Wu, Wang, Yang, and co-workers (Scheme 1a).8 Recently, Curran reported an Ir-catalyzed 1,4-hydroboration of electron-deficient arenes with NHC–BH3 under CFL irradiation (Scheme 1b).9 Very recently, Wang, Liang, and Zhu disclosed a photoinduced regio- and stereoselective fluorocarboborylation of alkenes.10 However, direct C–H borylation of N-heterocycles especially for indolizines utilizing NHC–boranes have not been successfully developed.
Scheme 1. Visible light-induced borylation with NHC–BH3.
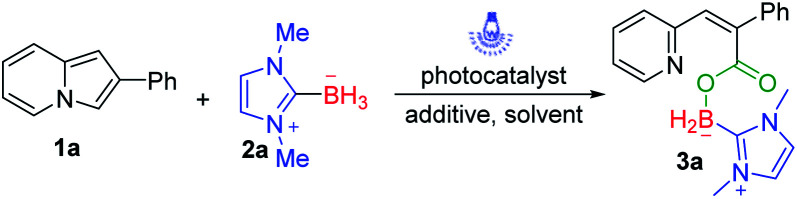
Indolizines, which contain 10 π-electrons, are isoelectronic with indole. So, they represent an important class of N-heterocycles and are widely applied in the field of material science.11 We have long been committed to developing green and sustainable new methods for C–H bond functionalization of indolizines to synthesize indolizines derivatives.12 In 2019, we disclosed visible-light-induced intermolecular [3 + 2] alkenylation–cyclization of indolizines to obtain pyrrolo[2,1,5-cd]indolizines.13 Very recently, we successfully constructed pyrrolo[2,1,5-cd]indolizine rings via visible-light-induced intermolecular [3 + 2] cycloaddition of indolizines and alkynes.14 Considering the great value of organoboron compounds, we expected that direct C–H borylation of indolizines with NHC–boranes would be realized via a clean photoredox system (Scheme 1c). However, the desired borylated indolizines were not observed via photoinduced C(sp2)–H borylation of indolizines and NHC–boranes. It turned out that sequence photooxygenation–borylation of indolizines occurred, delivering the deconstructive NHC–boryl carboxylates, and this type of borane compound is rarely reported.15 Herein, we disclose our unexpected findings that successive oxidative ring-opening and borylation of indolizines with NHC–boranes was accomplished under the irradiation of visible light. A variety of NHC–boryl carboxylates could be afforded through this manner (Scheme 1c).
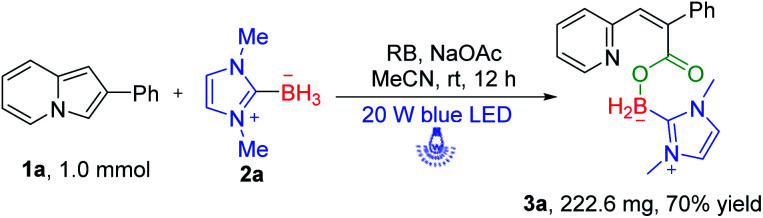
To optimize the conditions, we employed 2-phenylindolizine 1a and NHC–borane 2a as the model substrates for reaction development (Table 1). Initially, rose bengal (RB) was used as the photosensitizer in MeCN. The unexpected product 3a was obtained in 36% yield after reaction proceeded at room temperature for 12 h with a 20 W blue LED (455 nm) (Table 1, entry 1). After this, we tried to optimize the reaction using other photosensitizers. Eosin Y, rhodamine 6G, fluorescein, and Ir(ppy)3 were investigated, and the results indicated that none of them promoted this reaction better than RB under blue LED irradiation (Table 1, entries 2–5).
Optimization of the reaction conditionsa.
| ||||
|---|---|---|---|---|
| Entry | Photocatalyst | Additive | Solvent | Yieldb (%) |
| 1 | Rose bengal | — | CH3CN | 36 |
| 2 | Eosin Y | — | CH3CN | 18 |
| 3 | Rhodamine 6G | — | CH3CN | n. d. |
| 4 | Fluorescein | — | CH3CN | n. d. |
| 5 | Ir(ppy)3 | — | CH3CN | 23 |
| 6 | Rose bengal | Cs2CO3 | CH3CN | 51 |
| 7 | Rose bengal | K2CO3 | CH3CN | 38 |
| 8 | Rose bengal | DBU | CH3CN | 21 |
| 9 | Rose bengal | DABCO | CH3CN | 14 |
| 10 | Rose bengal | NaOAc | CH 3 CN | 72 |
| 11 | Rose bengal | NaOAc | THF | 68 |
| 12 | Rose bengal | NaOAc | DCE | 60 |
| 13 | Rose bengal | NaOAc | Toluene | 45 |
| 14 | Rose bengal | NaOAc | DMSO | 51 |
| 15 | — | NaOAc | CH3CN | n. d. |
| 16c | Rose bengal | NaOAc | CH3CN | n. d. |
| 17d | Rose bengal | NaOAc | CH3CN | 70 |
| 18e | Rose bengal | NaOAc | CH3CN | n. d. |
| 19f | Rose bengal | NaOAc | CH3CN | n. d. |
| 20g | Rose bengal | NaOAc | CH3CN | n. d. |
Standard conditions: 1a (0.2 mmol), 2a (0.4 mmol), photocatalyst (5 mol%), additive (0.4 mmol), solvent (2 mL), and irradiation with a 20 W blue LED for 12 h, air, n. d. = not detected.
Isolated yields.
Without light.
Under O2.
Under N2.
CuI (5 mol%).
Cu(OAc)2 (5 mol%).
Then, we turned our attention to examining the effect of different additives, such as Cs2CO3, K2CO3, DBU, DABCO, and NaOAc (Table 1, entries 6–10). Among them, NaOAc gave the best result, providing the target NHC–boryl carboxylate 3a in 72% yield (Table 1, entry 10). Encouraged by this result, we next tried to test the reaction using various solvents (Table 1, entries 10–14). It was found that only MeCN gives the target product 3a in good yield (entry 10), and other solvents led to lower yield. In addition, we verified that the product was not obtained without photosensitizer and light (Table 1, entries 15 and 16). We then examined the effect of the atmosphere on this ring-opening and borylation reaction. After filling the reaction with N2 and O2, respectively, the results show that reactions performed at O2 atmosphere produce product 3a normally but those filled with N2 do not (Table 1, entries 17 and 18). We can hardly observe the product in the presence of Cu(i) or Cu(ii) salts (Table 1, entries 19 and 20). Thus, the optimal reaction conditions were achieved by adding 5 mol% RB and 2 equiv. of NaOAc in MeCN at room temperature for 12 h under 20 W blue LED.
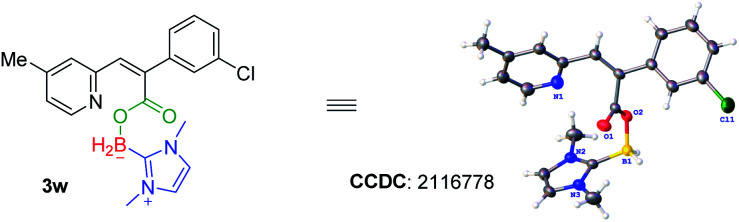
With a set of optimized reaction conditions in hand, we next examined the substrate scope with respect to various indolizine derivatives (Scheme 2). The reaction proceeded well under the standard reaction conditions and afforded the corresponding NHC–boryl carboxylates 3a–3aa in 56–75% yields. For example, alkyl substituents (–Me or –Et) and electron-donating substituents (–OMe), electron-withdrawing substituents (–Br) substituted on the indolizine ring did not affect the reactivity, and they were effective to afford NHC–boryl carboxylates under the optimized conditions (3b–3g). A series of various functional groups substituted on the ortho, para and meta position of benzene ring were compatible too (3h–3r). We next tested the reactivity of the substrates that are dichlorosubstituted on benzene rings, and the desired products 3s and 3t were obtained in 70% and 72% yields. Furthermore, when the model substrate 1a was replaced by 2-(4-fluorophenyl)-8-methylindolizine 1u, 2-(4-chlorophenyl)-8-methylindolizine 1v, and their analogues(1w–1z), respectively, the corresponding products (3u–3z) were obtained in good yields as well. The molecular structure of the product 3w was determined by X-ray crystallography (CCDC number: 2116778) (Scheme 3). In addition, the reaction of 2-(naphthalen-2-yl)indolizine 1aa with 2a was also performed to give the desired NHC–boryl carboxylate 3aa in 70% yield.
Scheme 2. Substrate scope of 2-phenylindolizinea. aReaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), RB (5 mol%), NaOAc (0.4 mmol), MeCN = 2 mL, irradiation with a 20 W blue LED for 12 h, air, isolated yield.
Scheme 3. Crystal structure of compound 3w.
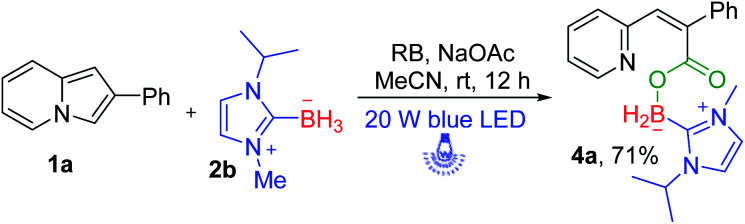
Next, we further turned our attention to exploring the NHC–borane of this photoinduced successive oxidative ring-opening and borylation reaction. We employed (1-isopropyl-3-methyl-1H-imidazol-3-ium-2-yl) trihydroborate 2b instead of 2a to participate in photoinduced transformation (Scheme 4). It was pleased to find that the desired NHC–boryl carboxylate 4a was obtained in 71% yield under the standard reaction conditions.
Scheme 4. Substrate scope of NHC–boranea. aReaction conditions: 1a (0.2 mmol), 2b (0.4 mmol), RB (5 mol%), NaOAc (0.4 mmol), MeCN (2 mL), and irradiation with a 20 W blue LED for 12 h. Isolated yield.
After the substrate scope exploration of this transformation, the scale-up experiment was studied. We enlarged the model substrate 1a to 1.0 mmol with 2a (2 mmol) under the irradiation with a 20 W blue LED for 12 h, and we found that boryl carboxylate product 3a was still produced though the yield has a small decrease (49%) (Scheme 5).
Scheme 5. Scale-up experimenta. aReaction conditions: 1a (1.0 mmol), 2a (2.0 mmol), RB (5 mol%), NaOAC (2 mmol), MeCN (10 mL), and irradiation with a 20 W blue LED for 12 h.
To gain some insight into the mechanism of this transformation, several controlled experiments were designed and carried out (Scheme 6). The yield of 3a dropped sharply when the reaction of 1a with 2a was conducted in the presence of a radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT).
Scheme 6. Control experiment.
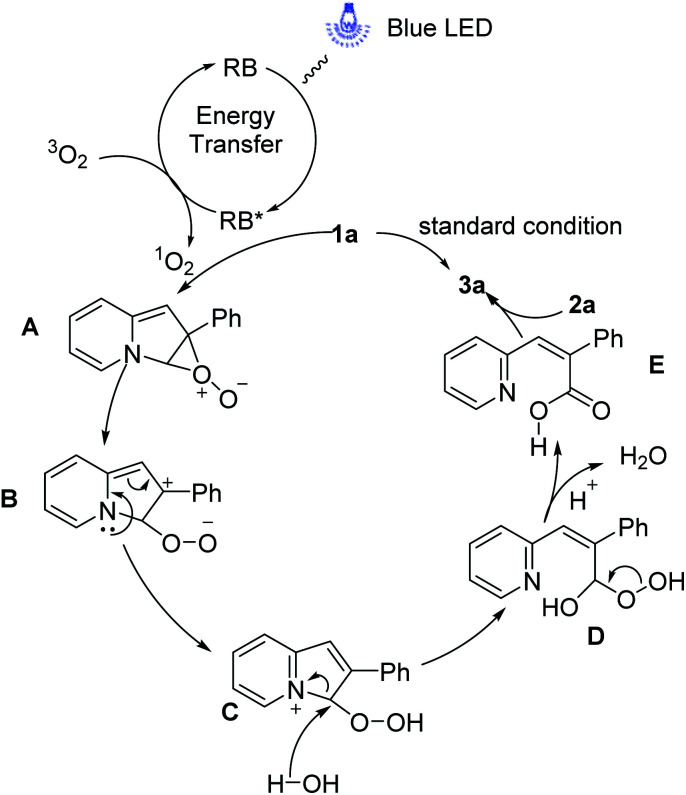
Based on our experimental results and literature reports,13,16 the possible mechanisms for this reaction of the ring-opening and borylation of indolizines have been depicted in Scheme 7. Under visible-light irradiation, the ground state of RB is converted to the excited RB* via the energy transfer, which interacts with 3O2 to give the 1O2 and RB to realize the photoredox cycle. In the presence of singlet oxygen, 1a readily forms the perepoxide exciplex A which is easily transferred to peroxidic zwitterion intermediate B. Subsequently, the intermediate B produced the intermediate C by intramolecular electron transfer. Then the trace amount of water in the solvent attack at C3 of intermediate C and cleavage the C–N bond to give the intermediate D, which would get rid of a molecule of water to generate acrylic acid intermediate E. Finally, the intermolecular reaction of acrylic acid intermediate E to NHC–borane 2a furnishes the target product 3a.
Scheme 7. Plausible mechanistic pathway.
Conclusions
In summary, we have developed an environmentally benign visible-light-induced ring-opening and borylation of indolizines strategy, which is benign using visible light as energy source and air as oxidant. A broad range of ring-opening and borylation of indolizines derivatives were conveniently synthesized in moderate to good yields with good functional group tolerance. Furthermore, other ring-opening reactions of indolizines are ongoing and will be reported in the near future.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (22001045), the Innovation and Strong School Project of Guangdong Pharmaceutical University (2021ZDZX2028), Guangdong Cosmetics Engineering & Technology Research Center, Provincial Experimental Teaching Demonstration Center of Chemistry & Chemical Engineering, Special funds of key disciplines construction from Guangdong and Zhongshan cooperating.
Electronic supplementary information (ESI) available. CCDC 2116778. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra08072e
Notes and references
- (a) Neeve E. C. Geier S. J. Mkhalid I. A. I. Westcott S. A. Marder T. B. Chem. Rev. 2016;116:9091–9161. doi: 10.1021/acs.chemrev.6b00193. [DOI] [PubMed] [Google Scholar]; (b) Cuenca A. B. Shishido R. Ito H. Fernandez E. Chem. Soc. Rev. 2017;46:415–430. doi: 10.1039/C6CS00692B. [DOI] [PubMed] [Google Scholar]; (c) Mkhalid I. A. I. Barnard J. H. Marder T. B. Murphy J. M. Hartwig J. F. Chem. Rev. 2010;110:890–931. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
- (a) Miyaura N. Suzuki A. Chem. Rev. 1995;95:2457. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]; (b) Crudden C. M. Glasspoole B. W. Lata C. J. Chem. Commun. 2009;45:6704. doi: 10.1039/B911537D. [DOI] [PubMed] [Google Scholar]; (c) Yang J. Deng M. Yu T. Chin. J. Org. Chem. 2013;33:693. doi: 10.6023/cjoc201303005. [DOI] [Google Scholar]; (d) Hall D. G., Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, Wiley-VCH, Weinheim, 2nd edn, 2011 [Google Scholar]
- (a) Fyfe J. W. B. Watson A. J. B. Chem. 2017;3:31–55. doi: 10.1016/j.chempr.2017.05.008. [DOI] [Google Scholar]; (b) Contemporary Boron Chemistry, ed. M. G. Davidson, K. Wade, T. B. Marder and A. K. Hughes, Royal Society of Chemistry, 2007 [Google Scholar]
- (a) Curran D. P. Solovyev A. Makhlouf Brahmi M. Fensterbank L. Malacria M. Lacôte E. Angew. Chem., Int. Ed. 2011;50:10294. doi: 10.1002/anie.201102717. [DOI] [PubMed] [Google Scholar]; (b) Walton J. C. Angew. Chem., Int. Ed. 2009;48:1726. doi: 10.1002/anie.200805362. [DOI] [PubMed] [Google Scholar]; (c) Roberts B. P. Chem. Soc. Rev. 1999;28:25. doi: 10.1039/A804291H. [DOI] [Google Scholar]; (d) Taniguchi T. Chem. Soc. Rev. 2021;50:8995. doi: 10.1039/D1CS00385B. [DOI] [PubMed] [Google Scholar]
- (a) Takahashi K. Geib S. J. Maeda K. Curran D. P. Taniguchi T. Org. Lett. 2021;23:1071–1075. doi: 10.1021/acs.orglett.0c04284. [DOI] [PubMed] [Google Scholar]; (b) Jin J. Zhang F. Zhao Q. Lu J. Wang Y. Org. Lett. 2018;20:7558–7562. doi: 10.1021/acs.orglett.8b03303. [DOI] [PubMed] [Google Scholar]; (c) Huang Y. Wang J. Zheng W. Zhang F. Yu Y. Zheng M. Zhou X. Wang Y. Chem. Commun. 2019;55:11904–11907. doi: 10.1039/C9CC06506G. [DOI] [PubMed] [Google Scholar]; (d) Shimoi M. Watanabe T. Maeda K. Curran D. P. Taniguchi T. Angew. Chem., Int. Ed. 2018;57:9485–9490. doi: 10.1002/anie.201804515. [DOI] [PubMed] [Google Scholar]
- (a) Tian Y. Guo X. Braunschweig H. Radius U. Marder T. B. Chem. Rev. 2021;121:3561–3597. doi: 10.1021/acs.chemrev.0c01236. [DOI] [PubMed] [Google Scholar]; (b) Kim J. H. Constantin T. Simonetti M. Llaveria J. Sheikh N. S. Leonori D. Nature. 2021;595:677–683. doi: 10.1038/s41586-021-03637-6. [DOI] [PubMed] [Google Scholar]
- (a) Zhu C. Dong J. Liu X. Gao L. Zhao Y. Xie J. Li S. Zhu C. Angew. Chem., Int. Ed. 2020;59:12817–12821. doi: 10.1002/anie.202005749. [DOI] [PubMed] [Google Scholar]; (b) Zhou N. Yuan X. A. Zhao Y. Xie J. Zhu C. Angew. Chem., Int. Ed. 2018;57:3990–3994. doi: 10.1002/anie.201800421. [DOI] [PubMed] [Google Scholar]
- (a) Xu W. Jiang H. Leng J. Ong H. Wu J. Angew. Chem., Int. Ed. 2020;59:4009–4016. doi: 10.1002/anie.201911819. [DOI] [PubMed] [Google Scholar]; (b) Xia P. Ye Z. Hu Y. Xiao J. Chen K. Xiang H. Chen X. Yang H. Org. Lett. 2020;22:1742–1747. doi: 10.1021/acs.orglett.0c00020. [DOI] [PubMed] [Google Scholar]
- Dai W. Geib S. Curran D. J. Am. Chem. Soc. 2020;142:6261–6267. doi: 10.1021/jacs.0c00490. [DOI] [PubMed] [Google Scholar]
- (a) Jin J. Zheng W. Xia H. Zhang F. Wang Y. Org. Lett. 2019;21:8414–8418. doi: 10.1021/acs.orglett.9b03173. [DOI] [PubMed] [Google Scholar]; (b) Xia P. Song D. Ye Z. Hu Y. Xiao J. Xiang H. Chen X. Yang H. Angew. Chem., Int. Ed. 2020;59:6706–6710. doi: 10.1002/anie.201913398. [DOI] [PubMed] [Google Scholar]; (c) Qi J. Zhang F. Jin J. Zhao Q. Li B. Liu L. Wang Y. Angew. Chem., Int. Ed. 2020;59:12876–12884. doi: 10.1002/anie.201915619. [DOI] [PubMed] [Google Scholar]
- (a) Sung J. Lee Y. Cha J. H. Park S. B. Kim E. Dyes Pigm. 2017;145:461–468. doi: 10.1016/j.dyepig.2017.06.014. [DOI] [Google Scholar]; (b) Ge Y. Liu A. Dong J. Duan G. Cao X. Li F. Sens. Actuators. 2017;247:46–52. doi: 10.1016/j.snb.2017.02.157. [DOI] [Google Scholar]; (c) Mitsumori T. Bendikov M. Dautel O. Wudl F. Shioya T. Sato H. Sato Y. J. Am. Chem. Soc. 2004;126:16793–16803. doi: 10.1021/ja049214x. [DOI] [PubMed] [Google Scholar]; (d) Shen Y. M. Grampp G. Leesakul N. Hu H. W. Xu J.-H. Eur. J. Org. Chem. 2007:3718–3726. doi: 10.1002/ejoc.200700250. [DOI] [Google Scholar]; (e) Gopal M. S. Anitha I. Jesny S. Menon S. J. Lumin. 2016;179:361–365. doi: 10.1016/j.jlumin.2016.04.039. [DOI] [Google Scholar]; (f) Huckaba A. J. Giordano F. McNamara L. E. Dreux K. M. Hammer M. I. Tschumper G. S. Zakeeruddin S. M. Grätzel M. Nazeeruddin M. K. Delcamp J. H. Adv. Energy Mater. 2015;5:1401629. doi: 10.1002/aenm.201401629. [DOI] [Google Scholar]; (g) Huckaba A. J. Yella A. Brogdon P. Murphy J. S. Nazeeruddin M. K. Grätzel M. Delcamp J. H. Chem. Commun. 2016;52:8424–8427. doi: 10.1039/C6CC02247B. [DOI] [PubMed] [Google Scholar]; (h) Huckaba A. J. Yella A. McNamara L. E. Steen A. E. Murphy J. S. Carpenter C. A. Puneky G. D. Hammer N. I. Nazeeruddin M. K. Grätzel M. Delcamp J. H. Chem.–Eur. J. 2016;22:15536–15542. doi: 10.1002/chem.201603165. [DOI] [PubMed] [Google Scholar]; (i) Kim E. Lee Y. Lee S. Park S. B. Acc. Chem. Res. 2015;48:538–547. doi: 10.1021/ar500370v. [DOI] [PubMed] [Google Scholar]
- (a) Song D. Huang C. Liang P. Zhu B. Liu X. Cao H. Org. Chem. Front. 2021;8:2583. doi: 10.1039/D1QO00224D. [DOI] [Google Scholar]; (b) Liu X. Song D. Zhang Z. Lin J. Zhuang C. Zhan H. Cao H. Org. Biomol. Chem. 2021;19:5284. doi: 10.1039/D1OB00701G. [DOI] [PubMed] [Google Scholar]; (c) Li J. Liu X. Deng J. Huang Y. Pan Z. Yu Y. Cao H. Chem. Commun. 2020;56:735–738. doi: 10.1039/C9CC08784B. [DOI] [PubMed] [Google Scholar]
- Liang Y. Teng L. Wang Y. He Q. Cao H. Green Chem. 2019;21:4025. doi: 10.1039/C9GC01766F. [DOI] [Google Scholar]
- Zhang Y. Yu Y. Liang B. Pei Y. Liu X. Yao H. Cao H. J. Org. Chem. 2020;85:10719–10727. doi: 10.1021/acs.joc.0c01253. [DOI] [PubMed] [Google Scholar]
- Bolt D. Curran D. Adv. Synth. Catal. 2020;362:2238–2244. doi: 10.1002/adsc.202000147. [DOI] [Google Scholar]
- (a) Tian J. Zhang Z. Yang X. Fun H. Xu J. J. Org. Chem. 2001;66:8230–8235. doi: 10.1021/jo015769o. [DOI] [PubMed] [Google Scholar]; (b) Li Y. Hu H. Ye J. Fun H. Hu H. Xu J. J. Org. Chem. 2004;69:2332–2339. doi: 10.1021/jo035070d. [DOI] [PubMed] [Google Scholar]; (c) Solovyev A. Chu Q. Geib J. Fensterbank L. Malacria M. Lacôte E. Curra D. P. J. Am. Chem. Soc. 2010;132:15072–15080. doi: 10.1021/ja107025y. [DOI] [PubMed] [Google Scholar]; (d) Willcox D. R. Nichol G. S. Thomas S. P. ACS Catal. 2021;11:3190–3197. doi: 10.1021/acscatal.1c00282. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.