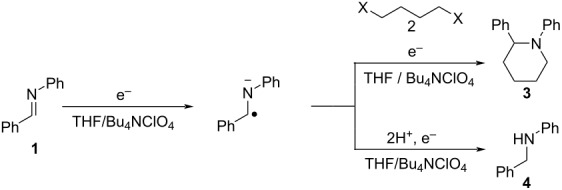
Table 7.
Yields of 3a and 4 in the model reductive cyclization using dihaloalkanes with different types of terminal halogensa.
| |||
|
| |||
| Entry | Type of X | 3ab (%) | 4b (%) |
|
| |||
| 1 | Br (2a) | 78 | 11 |
| 2 | Cl (2b) | 6 | 72 |
| 3 | I (2c) | 14 | 13 |
aExperimental conditions: cathode, GC plate; anode, Pt plate; electricity, 2.15 F mol−1; current density, 12.7 mA cm−2; electrode distance, 40 μm; solvent, THF; substrate, 0.06 M benzylideneaniline (1) and 0.12 M 1,4-dihaloalkane (2a, 2b, or 2c); base added, 0.06 M DBU; supporting electrolyte, 0.14 M n-Bu4N∙ClO4; flow rate, 11 mL h−1 (residence time, 3.9 s). bDetermined by HPLC.
