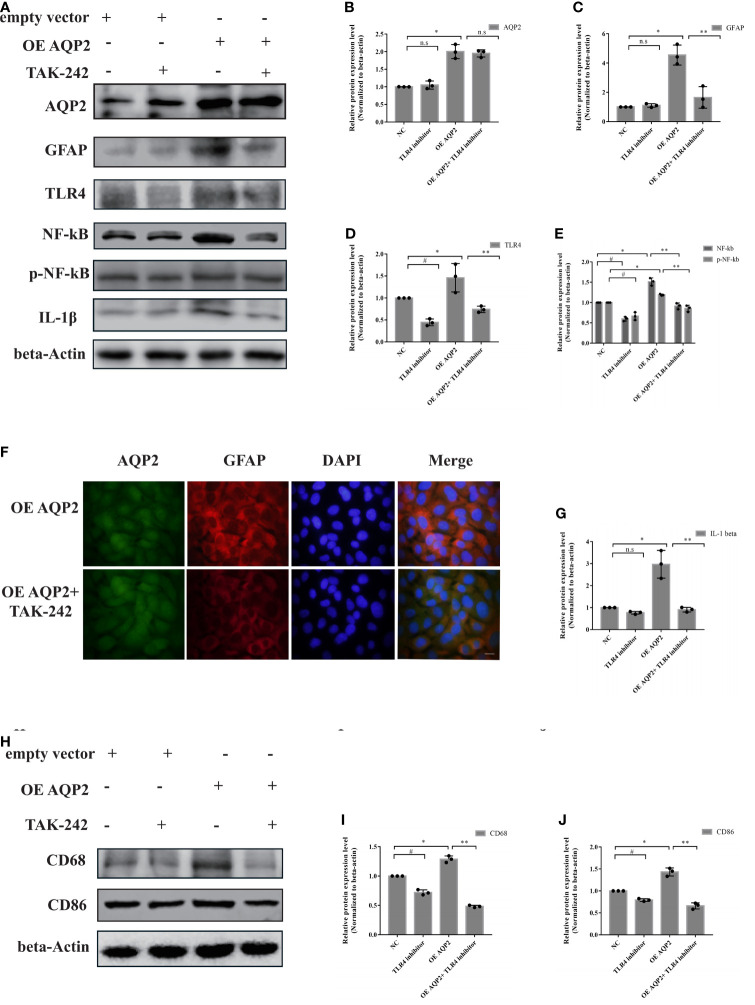
Figure 6.
AQP2 overexpression-induced pro-inflammation secretion is partially caused by TLR4 signaling activation in astrocyte. Astrocyte with overexpressed AQP2 (pcDNA3.1-AQP2 plasmid) were exposed to TLR inhibitor (TAK-242) for 24 h. (A) Western blot analysis of AQP2, GFAP, TLR4, NF-κB, phospho- NF-κB and IL-1β in astrocyte transfected with pcDNA3.1 empty vector or pcDNA3.1-AQP2 plasmid treated with or without TAK-242 (n = 3/group). (B–E, G) Quantification of the relative protein expression levels of AQP2, GFAP, TLR4, NF-kB and IL-1β. (#p < 0.05, empty vector + vehicle vs. empty vector+ TAK-242; *p < 0.05, empty vector + vehicle vs. pcDNA3.1-AQP2 plasmid + vehicle; **p < 0.05, pcDNA3.1-AQP2 plasmid + vehicle vs. pcDNA3.1-AQP2 plasmid + TAK-242). The data are presented as the mean ± s.d. of three independent experiments. (F) Immunofluorescence of AQP2 (red signal) and GFAP (green signal) in astrocyte with AQP2 overexpression treated with or without TAK-242. Astrocyte cell nuclei were immunostained (blue, DAPI). Scale bar: 10 μm. (H) Microglia were treated with medium of astrocyte for 24h transfected with pcDNA3.1 empty vector or pcDNA3.1-AQP2 plasmid (with or without TAK-242). CD68 and CD86 were assessed by western blotting (n = 3/group). Quantification of the relative protein expression level of CD68 (I) and CD86 (J) is shown. (#p < 0.05, empty vector + vehicle vs. empty vector+ TAK-242; * p < 0.05, empty vector + vehicle vs. pcDNA3.1-AQP2 plasmid + vehicle; **p < 0.05, pcDNA3.1-AQP2 plasmid + vehicle vs. pcDNA3.1-AQP2 plasmid + TAK-242). The data are presented as the mean ± s.d. of three independent experiments.