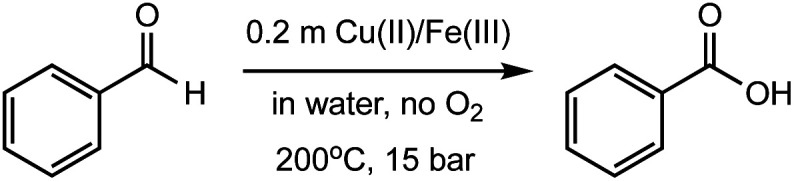
Investigation of oxidation of benzaldehyde using copper(ii) and iron(iii) salts at different reaction conditions.
| ||||
|---|---|---|---|---|
| Entry | Condition | Additive | Conversion | Benzoic acid yielda |
| 1 | 200 °C, 24 h | None | 4% | 3% |
| 2 | 200 °C, 24 h | 0.2 m CuCl2 | 8% | 7% |
| 3 | 200 °C, 24 h | 0.2 m CuSO4 | 9% | 8% |
| 4 | 200 °C, 24 h | 0.2 m Cu(OAc)2 | 6% | 5% |
| 5 | 200 °C, 0.5 h | 0.2 m Cu(NO3)2 | 12% | 10% |
| 6 | 200 °C, 1 h | 0.2 m Cu(NO3)2 | 25% | 24% |
| 7 | 200 °C, 2 h | 0.2 m Cu(NO3)2 | 30% | 26% |
| 8 | 200 °C, 2 h | 0.1 m Cu(NO3)2 + 0.1 m NaNO3 | 15% | 13% |
| 9 | 200 °C, 2 h | 0.2 m NaNO3 | 11% | 10% |
| 10 | 200 °C, 24 h | 0.2 m FeCl3 | 14% | 13% |
| 11 | 200 °C, 24 h | 0.1 m Fe2(SO4)3 | 11% | 10% |
| 12 | 100 °C, 2 h | 0.2 m Fe(NO3)3 | 39% | 38% |
| 13 | 140 °C, 2 h | 0.2 m Fe(NO3)3 | 59% | 57% |
| 14 | 170 °C, 1 h | 0.2 m Fe(NO3)3 | 62% | 59% |
| 15 | 200 °C, 0.5 h | 0.2 m Fe(NO3)3 | 64% | 60% |
| 16 | 200 °C, 1 h | 0.2 m Fe(NO3)3 | >99% | 94% |
| 17 | 200 °C, 2 h | 0.2 m Fe(NO3)3 | >99% | 98% |
| 18 | 200 °C, 2 h | 0.1 m Fe(NO3)3 | >99% | 90% |
| 19 | 200 °C, 2 h | 0.2 m FeCl3 + 0.2 m NaNO3 | >99% | 97% |
| 20 | 200 °C, 2 h | 0.2 m FeCl3 + 0.6 m NaNO3 | >99% | 98% |
| 21 | 200 °C, 2 h | 0.2 m Mg(NO3)2 | 23% | 21% |
| 22 | 200 °C, 2 h | 0.2 m Ca(NO3)2 | 9% | 8% |
Yield determined by gas chromatography.
