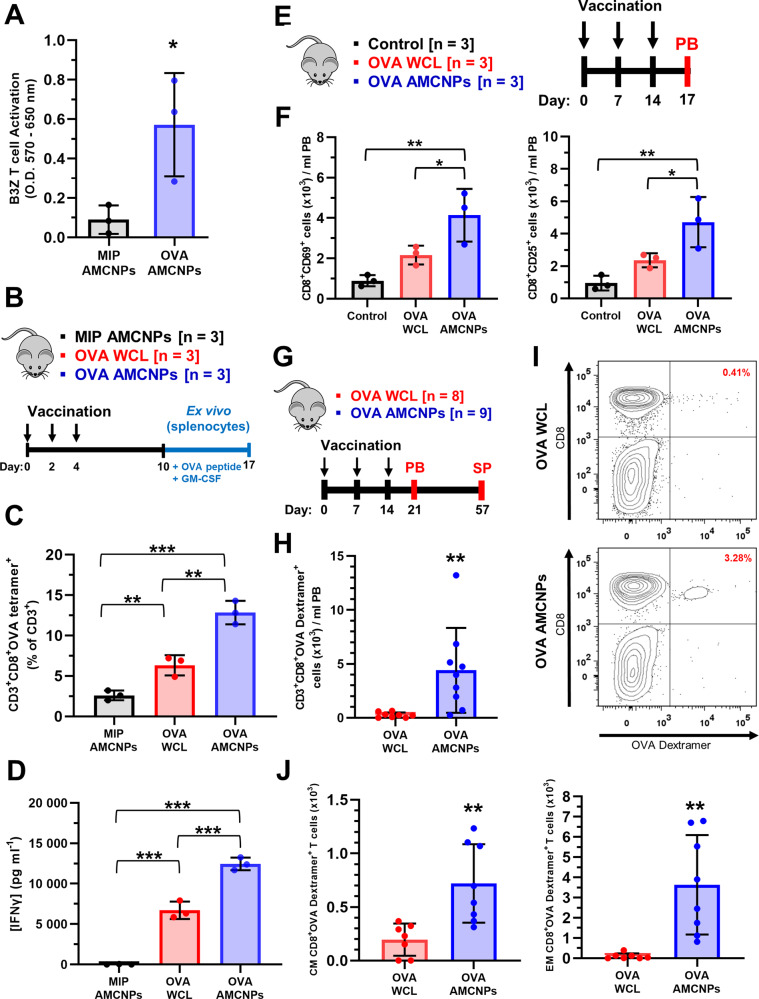
Fig. 5. AMCNPs enhance antigen-specific T cell activation.
A BMDCs were pulsed with C1498-MIP AMCNPs or C1498-OVA AMCNPs before co-culture with B3Z CD8+ T cells. OVA-specific B3Z CD8+ T cell activation was measured by a CPRG assay. Data shown as mean optical density at 570–650 nm from three experiments. Significance was determined by unpaired t-test. B–D Mice were vaccinated 3 times as indicated, with C1498-MIP AMCNPs, equivalent C1498-OVA WCL vaccine, or C1498-OVA AMCNPs; splenocytes were collected and re-stimulated ex vivo with OVA SIINFEKL peptide for 7 days (n = 3). C OVA-specific T cell expansion was measured by H-2Kb:SIINFEKL tetramer staining of CD3+CD8+ T cells. Data is presented as the mean frequency of CD3+CD8+OVA tetramer+ cells among CD3+ cells. D The concentration of secreted IFN-γ was measured by ELISA. C, D Significance was determined using one-way ANOVA with a post-hoc test using the Holm-Šídák method. E, F Mice were vaccinated 3 times, as indicated, with C1498-OVA AMCNPs (n = 3), equivalent C1498-OVA WCL vaccine (n = 3), or mock vaccination control (n = 3). Total number of CD69+ or CD25+ CD8+ T cells among peripheral blood (PB) mononuclear cells was determined by flow cytometry on day 17 and normalized to 1 ml of PB. Significance was determined using one-way ANOVA with a post-hoc test using the Holm-Šídák method. G–J Mice were vaccinated 3 times, as indicated, with C1498-OVA AMCNPs (n = 9) or equivalent C1498-OVA WCL vaccines (n = 8). H, I OVA-specific T cell expansion was determined through staining with H-2Kb:SIINFEKL dextramer (OVA-dextramer) of PB mononuclear cells on day 21. H Total CD3+CD8+OVA dextramer+ events observed were normalized to 1 ml of PB and adjusted for background staining by subtracting the average number of events in unvaccinated controls (n = 5). Significance was determined using one-way ANOVA with a post-hoc test using the Holm-Šídák method. I Representative flow cytometry plots of CD3+ gated live cells used to quantify CD3+CD8+OVA dextramer+ events are shown. J OVA-specific central memory (CM, CD62LhiCD44hiCD8+OVA dextramer+) and effector memory (EM, CD62LlowCD44hiCD8+OVA dextramer+) expansion was determined through flow cytometry of live splenocytes on day 57. Total events observed were normalized to the total number of live splenocytes collected and adjusted for background staining by subtracting the average number of events in unvaccinated controls (n = 5). Significance was determined using one-way ANOVA with a post-hoc test using the Holm-Šídák method.