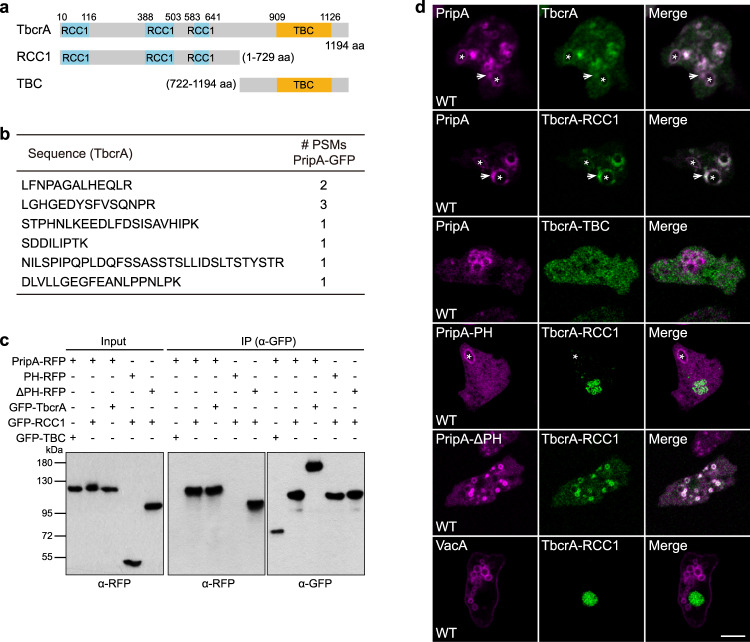
Fig. 6. PripA forms a complex with TbcrA.
a Schematic representation of TbcrA, TbcrA-RCC1, and TbcrA-TBC. b Proteomic identification of TbcrA as a binding partner of PripA. The table shows the identified unique peptides of TbcrA from PripA-GFP, but not GFP control, pull-down. c Co-immunoprecipitation experiments showing that PripA and PripA-∆PH specifically pulled down TbcrA and TbcrA-RCC1 from cell lysates. Data was from one representative experiment out of two independent experiments. d Co-expression of RFP-tagged PripA, PripA-PH, PripA-∆PH, or VacA with GFP-tagged TbcrA, TbcrA-RCC1, or TbcrA-TBC. The Pearson’s correlation coefficient was 0.94 ± 0.01 for PripA and TbcrA, 0.91 ± 0.03 for PripA and TbcrA-RCC1, 0.24 ± 0.03 for PripA and TbcrA-TBC, 0.33 ± 0.12 for PripA-PH and TbcrA-RCC1, 0.94 ± 0.03 for PripA-ΔPH and TbcrA-RCC1, and 0.09 ± 0.04 for VacA and TbcrA-RCC1. Data represents mean ± SEM (n = 10 cells). The asterisks mark newly formed macropinosomes and the arrows indicate surrounding vesicles. Scale bar = 5 μm. Source data for b, c are provided in this paper.