Abstract
The high cost for microalgae harvesting still is the bottleneck of microalgae commercial production. In the present study, the effect of adjusting pH to alkaline conditions with sodium hydroxide/calcium hydroxide and the addition of chitosan together with pH adjustments on the flocculation of Chlorella vulgaris (C. vulgaris) was studied, respectively. A single-factor experiment showed a maximum flocculation efficiency of 96.7% when adjusting the pH to 12 with calcium hydroxide. There was synergistic action between chitosan and calcium hydroxide. Flocculation conditions of C. vulgaris for the combined use of calcium hydroxide and chitosan was optimized by response surface methodology (RSM) with a Box-Behnken design (BBD). Flocculation efficiency reached 97.08% under optimal flocculation conditions when adjustion of pH to 8.97 with 2 g/L calcium hydroxide, a chitosan dosage of 20 mg/L, and a flocculation time of 60 min. The current study presents one method for efficient flocculation harvesting of C. vulgaris at weak alkaline conditions and low chitosan dosage.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-022-01004-1.
Keywords: Flocculation, Chlorella vulgaris, Calcium hydroxide, Chitosan, Response surface methodology
Introduction
Chlorella has received more attention as feedstock for health food, livestock feed, biofuels and pharmaceutics mainly for it is rich in protein, lipids, carbohydrates, pigments, vitamins and minerals [1, 2]. However, the harvesting cost accounts for more than 20% of the total cost of microalgae production and processing mainly because their small size (5~50μm) and low concentration (0.05~0.2g/L) is the bottleneck of microalgae commercial production [3].
Microalgae harvesting methods mainly include flocculation, filtration, centrifugation, and gravity sedimentation. Centrifugation harvesting microalgae is very expensive and high energy consuming, and poor performance of filtration and gravity sedimentation are caused by the small size and the colloid stability derived from the surface negative charge of microalgae [4]. Flocculation is a promising technology that permits low-cost and environmentally friendly harvesting microalgae on a commercial scale [5, 6]. Harvesting microalgae by flocculation mainly includes auto-flocculation, addition of chemical flocculants and bioflocculation. Auto-flocculation, the spontaneous flocculation of microalgae in culture, happens when the pH value exceeds 9.0 [7]. However, high alkaline might affect the downstream treatment and the quaility of microalgae [8]. Although chemical flocculants are effectively in harvesting microalgae, while the application of them might contaminate the biomass and influence the reuse of flocculation effluent [4]. Recently, ozone has been suggested as an alternative approach to simultaneously harvesting and disrupting C. vulgaris for biodiesel production [9]. Bioflocculation has been proved to be effectively and environmentally friendly [10]. However, it potentially poses a contamination risk [11].
Chitosan (a natural cationic polysaccharide) has been used to flocculation harvesting of microalgae mainly because it is harmless to the health of human [2]. However, the dosage and cost of chitosan are usually higher than those of chemical flocculants because of the low flocculation efficiency [12]. Vu et al. [13] reported the flocculation efficiency of chitosan was lower than 60 % for C. vulgaris even at a high dosage, and the flocculation efficiency increased 57% and 24% when using chitosan in combination with ferric chloride and aluminum sulfate, respectively. Kumaran et al. [14] reported the pH of the microalgae solution had a strongly influence on the flocculation of Chaetoceros muelleri at 80 mg/L chitosan. The flocculation efficiency was lower than 20% under acidic condition, while the efficiency significantly increased when the pH higher than 9.2, and the maximum flocculation efficiency reached 98.5±0.18% at pH 9.6. Loganathan et al. [15] reported the coagulation removal of microalgae from seawater with chitosan was weaker at alkaline than that in neutral condition, and the maximum removal efficiency reached 87.3% at optimal conditions. A higher than 97% microalgae removal efficiency was reached at the combined using of chitosan and ferric chloride/aluminum. The above studies indicated that the combined use of chitosan and metal ions could provide efficient coagulation of microalgae due to synergistic interactions.
Calcium hydroxide is more widely used as a coagulant agent and pH regulator mainly for its availability and low cost. To best of our knowledge, the combined used of calcium hydroxide and chitosan for the flocculation harvesting of C. vulgaris has rarely been studied. In the present study, the effect of adjusting pH with sodium hydroxide/calcium hydroxide and of the subsequent addition of chitosan on the flocculation of C. vulgaris was evaluated with single-factor experiments. Flocculation conditions for the combined use of chitosan and calcium hydroxide were optimized with response surface methodology (RSM). This study aims to devise an effective flocculation method to harvest C. vulgaris with a low dosage of chitosan and weak alkaline conditions.
Material and Methods
Cultivation of C. vulgaris
C. vulgaris used in the study was provided by Jiangxi Alga Biology technology Co., Ltd (Ruijin, Jiangxi province, china). C. vulgaris was cultured in modified BG-11 medium under a 12:12 h light/dark cycle with a light intensity of 5800 lx at 30 C.
Flocculation Experiments
The initial OD680 of C. vulgaris culture was adjusted to about 1.0 with deionized water. The pH values of the C. vulgaris culture were adjusted with diluted HCl or NaOH/Ca (OH)2 to the set values. The flocculation experiments were performed at the set pH values, chitosan concentrations, and set times. Flocculation experiments were performed in 50 mL beakers, stirred by magnetic stirrer for 3 min. Then, the culture was transferred into 30 mL graduated test tube, and settlement flocculation for the set time. In this study, three parallel tests were conducted, and the data used was the mean value of these three replicates.
Response Surface Methodology
RSM was used to optimize the flocculation condition by evaluating the significant and interactive influence of parameters on the flocculation efficiency (%) of C. vulgaris. Chitosan concentration, pH, and flocculation time were chosen as the main factors based on the results of single-factor experiments (see factor levels in Table 1). Seventeen tests were designed using a Box Behnken Design (BBD). The RSM experiments data and the validity of the proposed model were analyzed using design expert 8.06. The validity of the proposed model was verified by flocculation of C. vulgaris under the recommended conditions.
Table 1.
Range of the independent variables and their levels
| Independent variable | Variable | Factors level | ||
|---|---|---|---|---|
| − 1 | 0 | + 1 | ||
| pH | A | 7.5 | 8.25 | 9.0 |
| Chitosan concentration (mg/L) | B | 20 | 40 | 60 |
| Flocculation time (min) | C | 10 | 35 | 60 |
Analysis
Flocculation efficiency was determined through measuring the OD value of 680 nm of the supernatant taken from the middle of C. vulgaris suspension by UV spectrophotometer (UV 6000 Shimadzu; Ermington, NSW, Australia) before and after flocculation. Flocculation efficiency was calculated according to Eq. (1):
| 1 |
where OD680i and OD680f representing the optical density at 680 nm before and after flocculation, respectively.
Results and Discussion
Single-Factor Experiment
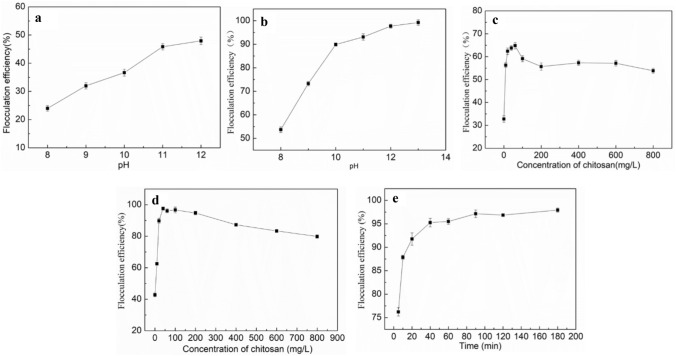
The effect of adjusting pH to alkaline conditions with sodium hydroxide/calcium hydroxide on C. vulgaris auto-flocculation was evaluated and the result is shown in Fig. 1a and b, respectively. The flocculation efficiency kept increased from 23.99 to 47.94% in the pH range of 8 to 12 (Fig. 1a). Maximum flocculation efficiency lower than 50% indicated the effect of sodium hydroxide was poor. The result is consistent with some studies. Sanyano et al.[16] reported the flocculation efficiency of marine Chlorella sp with the pH adjusted to 10.5 was lower than 50%. Pishgar et al. [12] found the maximum efficiency of Chlorella vulgaris was just 66% at pH 12.
Fig. 1.
Flocculation behaviors of C. vulgaris. pH adjusted with sodium hydroxide (a) and calcium hydroxide (b), chitosan individually (c), chitosan when adjusting the pH with calcium hydroxide (d), and flocculation at 40 mg/L chitosan and pH adjusted to 8.5 with 2 g/L calcium hydroxide at different times (e)
When adjusting the pH with calcium hydroxide, the flocculation efficiency was far higher than with sodium hydroxide (Fig. 1a, b). Flocculation efficiency increased from 53.7 to 96.7% when the pH values increased from 8.0 to 12.0. The result is consistent with Pirwitz et al. [17], they found that adjusting of pH with calcium hydroxide seems more preferable to sodium hydroxide in the flocculation harvesting of Dunaliella. The difference between the adjustion of pH with sodium hydroxide and calcium hydroxide was due to the latter inducing autoflocculation and providing calcium ions that enhance the flocculation of microalgae through a bridging mechanism [18].
The flocculation of microalgae using chitosan is sensitive to the pH, and the optimal pH range is 7–9 [19]. The effect of chitosan on the flocculation of C. vulgaris at the pH was adjusted to 8.5 with sodium hydroxide and calcium hydroxide was studied, and the result is shown in Fig. 1c and d, respectively.
As shown in Fig. 1c, the flocculation efficiency rapidly increased with the elevation of chitosan concentration when it is no higher than 60 mg/L, while further increases in chitosan concentration inhibited the flocculation of C. vulgaris. Higher dosage chitosan led to the charge reversal /re-stabilization of C. vulgaris because the excessive chitosan resulted in the microalgae surface with positive charge hamper the flocculation [15]. The maximum flocculation efficiency 64.82% achieved at 60 mg/L showed that the flocculation of C. vulgaris by chitosan was insufficient. These results are consistent with the results of some studies [13, 15], they also found the maximum flocculation efficiency of C. vulgaris by chitosan was lower than 70%.
Figure 1d shows that the flocculation efficiency increased from 42.73 to 97.54% in the chitosan concentration range between 0 and 40 mg/L when adjusting the pH to 8.5 with calcium hydroxide and a higher dosage of chitosan also inhibited flocculation. The flocculation efficiency of chitosan and calcium hydroxide in combination was far higher than that of individual applications, which indicated a synergistic interaction between them. This result is consistent with that of Vu et al. [13], they also found there are synergistic interactions when flocculating microalga with combined chitosan and inorganic salts. The presence of chitosan can induce the entrapment and bridging of C. vulgaris cells, which can promote the aggregation of microalgae particles to form bigger flocs through charge neutralization by calcium hydroxide [15].
The influence of flocculation time was evaluated by flocculating of C. vulgaris for different time periods. As shown in Fig. 1e, the flocculation efficiency increased quickly at the beginning of flocculation. About 95.5% C. vulgaris were flocculated at the first 40 min. Then, the flocculation efficiency oscillated between in the range of 40 to 180 min. This result is consistent with other studies. Kumaran et al. [14] also found that more than 99% Chaetoceros muelleri settling occurred in the first 40 min, and flocculation efficiency did not increase with settling time after that. Zhu et al. [20] reported that more than 90% C. vulgaris flocculation in the first 10 min after applying aluminum sulfate and aluminum potassium sulfate. This was mainly due to the break of the steady state by charge neutralization of the cells surfaces and the bridging of small cells to form bigger flocs and sedimentation both increased with flocculation time before it reached the balance.
Single-factor experiment results confirmed synergistic interactions between chitosan and calcium hydroxide, and C. vulgaris could be effectively flocculation harvested under weak alkaline conditions and low dosages of chitosan.
Optimize Flocculation Conditions by the Combined Used of Chitosan and Calcium Hydroxide by Response Surfaces
The BBD matrix, the measured and predicted values of flocculation efficiency are presented in Supplementary Table 2. The second-order polynomial model obtained by fitting of the experiments data to predict the flocculation efficiency, and the model is given in Eq. (2).
| 2 |
where Y present the predicted Flocculation efficiency as in Supplementary Table 1, and A, B, and C was represented pH, chitosan concentration, and flocculation time, respectively.
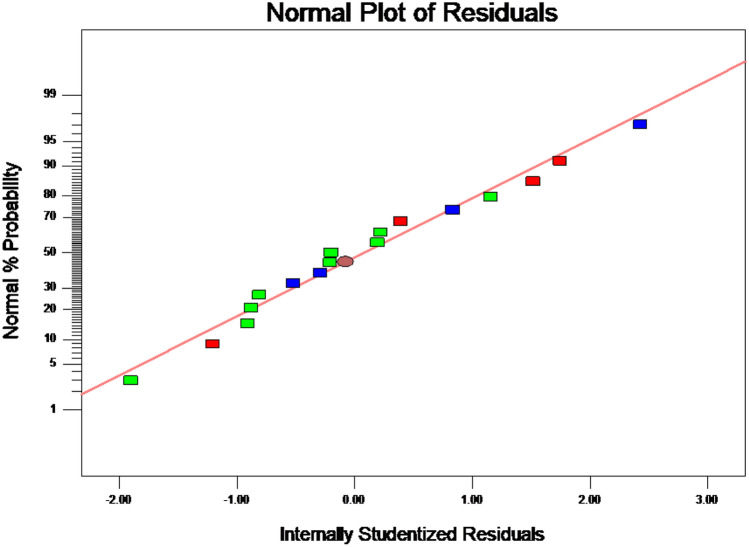
Analysis of variance (ANOVA) for the developed model (Eq. 2) are presented in Supplementary Table 2. The p-value of the model < 0.0001 and the high F value (2013.75) confirmed that the proposed second-order polynomial model was significant and suitable for the prediction of flocculation efficiency. The Lack of fit (LOF) > 0.05 indicated that the model was suitable and can be used to predict the flocculation efficiency [12]. In the present study, R2, the adjusted coefficient of determination (R2adj) and predicted R2 was 0.9996, 0.9991 and 0.9948, respectively. The high degree of agreement of these three values confirmed the model’s validity (Supplementary Table 2). The percent coefficient of variation (CV%) is an indicator of the reproducibility of the proposed model, and it should be less than 10. Here, the CV% was 1.11, confirming that the model had good reproducibility. Adequate precision is an indicator for the adequacy of the proposed models, and it should be higher than 4 for a good model [21]. In the present case, the adequate precision was 127.549, confirming that Eq. (2) could predict the flocculation efficiency and optimize the flocculation process parameters (Supplementary Table 1). As shown in Fig. 2, the residues were all on a straight line, confirming the constancy of the variance and normal distribution of data and that the model had good reliability and adequacy of the prediction of flocculation efficiency.
Fig. 2.
Normal probability plot of residuals
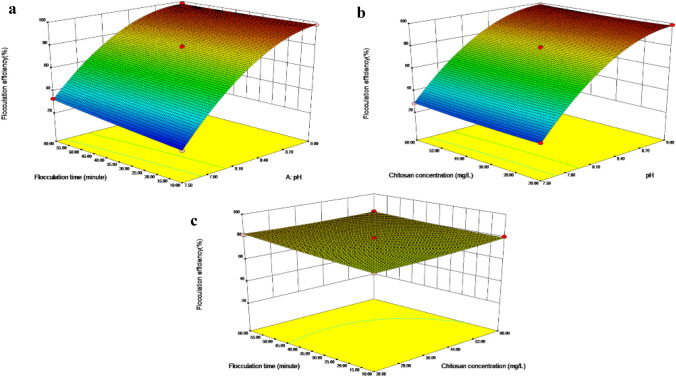
According to Supplementary Table 2, the linear terms (A, B and C), quadratic terms (A2, B2), and interactive terms (AC, BC) were significant for the flocculation of C. vulgaris because their p-values all far below 0.05. To further assess the interactions among variables (pH, chitosan concentration, and flocculation time), the response surface for the variables and flocculation efficiency is shown in Fig. 3.
Fig. 3.
Response surfaces and their corresponding contour plots for flocculation efficiency of C. vulgaris as a functional of a pH and flocculation time, b pH and chitosan concentration, and c chitosan concentration and flocculation time
Figure 3a and b show that the flocculation efficiency of C. vulgaris increased with the pH values, the flocculation efficiency of C. vulgaris increased from about 30% to nearly 100%, with pH increases from 7.5 to 9.0. This result was consistent with adjusting the pH with calcium hydroxide (Fig. 1b). The extension of the flocculation time promoted the flocculation of C. vulgaris, but the effect was weaker than that of pH (Fig. 3a, c). This result confirmed the extension of flocculation time favor of the flocculation of C. vulgaris (Fig. 1e). Figure 3b and c showed that the positive action of chitosan on the flocculation of C. vulgaris was weakest among the three factors, and the interaction effect between chitosan concentration and flocculation time was the weakest interaction. This result confirmed the result of flocculation under different chitosan concentration (Fig. 1c). Previous study have proved the effect of chitosan is unsatisfied even at a high dosage [13]. Figure 3 confirmed the influence sequence of the three variables on the flocculation is pH > flocculation time > chitosan concentration, which was consistent with the ANOVA analysis (Supplementary Table 2).
The optimize flocculation conditions proposed and the verification test results are shown in Table 2. The flocculation efficiency under the recommended conditions was 97.08%, which is very close to the value of 99.45% predicted by Eq. (2). Flocculation efficiency of Chaetoceros muelleri was higher than 99%, with an optimum dosage of chitosan (80 mg/L) at pH 9.6 and settling time of 40 min [14]. Loganathan et al. [15] studied the removal of microalgae from seawater by chitosan-alum and chitosan-ferric chloride. The maximum microalgae removal efficiencies using alum, chitosan, and FeCl3 individually as flocculants were 96.3, 87.3, and 98.6%, respectively. The maximum efficiencies reached 97.2 and 97.6% for dual coagulation using 5 ppm ferric chloride + 0.5 ppm chitosan and 10 ppm alum + 1 ppm chitosan, respectively. Zhu et al. [22] reported the lowest concentration of chitosan and aluminum sulfate required for the > 90% flocculation efficiency in C. vulgaris was 250 mg/L and 2500 mg/L respectively. Vu et al. [13] found 89% and 81% C. vulgaris was flocculated by 50 mg/L Al2(SO4)3 + 80 mg/L chitosan and 40 mg/L FeCl3 + 80 mg/L chitosan respectively. The maximum efficiency of 97.9% was reached at 100 mg/L bottom ash as flocculant, at 50 °C and pH 6.5 and 60 min flocculation time for C. vulgaris [23]. The flocculation performance in the present study was higher than in some studies [13, 23], near to the performance of another study [15], and slightly lower than in two other studies [14, 22]. Compared to these studies, flocculation was efficient and had the obvious advantages of lower chitosan dosages and weak alkaline conditions.
Table 2.
Optimum variables and response for flocculation efficiency
| pH | Chitosan concentration (mg/L) | Flocculation time (min) | Flocculation efficiency (%) measured | Flocculation efficiency (%) predicted |
|---|---|---|---|---|
| 8.97 | 20 | 60 | 97.08 | 99.45 |
Conclusions
The flocculation behavior of C. vulgaris when adjusting the pH with sodium hydroxide and calcium hydroxide showed that adjusting the pH to 12 with calcium hydroxide achieved maximum flocculation efficiency (96.7%). A maximum flocculation efficiency of C. vulgaris lower than 70% indicated that the effect of chitosan alone was poor. Flocculation of C. vulgaris with chitosan and calcium hydroxide in combination confirmed synergistic interactions. The flocculation conditions of C. vulgaris with chitosan and calcium hydroxide were optimized by RSM with BBD. The flocculation efficiency reached 97.08% when adjusting the pH to 8.97 with 2 g/L calcium hydroxide, chitosan concentrations of 20 mg/L, and flocculation times of 60 min. The results of the present study can provide theoretical directions for the design of calcium-chitosan flocculants through chemical modification of chitosan to simplify the efficient flocculation of microalgae and reduce the consumption of chitosan. The performance of calcium-chitosan in the flocculation harvesting of C. vulgaris should be further studied.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
CL: Conceptualization, Writing—Review & Editing and Funding acquisition. YY, YX, WY and JC: Data Curation, Investigation and Formal analysis. ZZ and XZ: Funding acquisition.
Funding
The work was supported by Primary Research & Development Plan of Jiangxi province (20171ACF60011, 20171ACF60008).
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khoo KS, Wen YC, Chew KW, Show PL. Microalgal-bacterial consortia as future prospect in wastewater bioremediation, environmental management and bioenergy production. Indian J Microbiol. 2021;61:262–269. doi: 10.1007/s12088-021-00924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira CDD, Rabello DME, Noseda MD. Biomass production and harvesting of Desmodesmus subspicatus cultivated in flat plate photobioreactor using chitosan as flocculant agent. J Appl Phycol. 2019;31:857–866. doi: 10.1007/s10811-018-1586-z. [DOI] [Google Scholar]
- 3.Zhu LD, Li ZH, Guo DB, Huang F, Xia K. Cultivation of Chlorella sp. with livestock waste compost for lipid production. Bioresour Technol. 2017;223:296–300. doi: 10.1016/j.biortech.2016.09.094. [DOI] [PubMed] [Google Scholar]
- 4.Show PL, Tan JS, Lee SY, Chew KW, Ho SH. A Review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered. 2020;11:116–129. doi: 10.1080/21655979.2020.1711626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei XQ, Chen Y, Shao ZG, Chen ZR, Li Y, Zhu H, Zhang JY, Zheng W, Zheng TL. Effective harvesting of the microalgae Chlorella vulgaris via flocculation-flotation with bioflocculant. Bioresource Technol. 2015;198:922–925. doi: 10.1016/j.biortech.2015.08.095. [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Xu YH, Sun WQ, Sun YJ, Zheng HD. UV-initiated synthesis of a novel chitosan-based flocculant with high flocculation efficiency for algal removal. Sci Total Environ. 2017;609:410–418. doi: 10.1016/j.scitotenv.2017.07.192. [DOI] [PubMed] [Google Scholar]
- 7.Tran NT, Seymour JR, Siboni N, Evenhuis CR, Tamburic B. Photosynthetic carbon uptake induces autoflocculation of the marine microalga Nannochloropsis oculata. Algal Res. 2017;26:302–311. doi: 10.1016/j.algal.2017.08.005. [DOI] [Google Scholar]
- 8.Wan C, Zhao XQ, Guo SL, Asraful Alam M, Bai FW. Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresource Technol. 2013;135:207–212. doi: 10.1016/j.biortech.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Wan NAK, Man KL, Uemura Y, Lim JW, Lee KT. Simultaneous harvesting and cell disruption of microalgae using ozone bubbles: optimization and characterization study for biodiesel production. Front Chem Sci Eng. 2021;15:1257–1268. doi: 10.1007/s11705-020-2015-9. [DOI] [Google Scholar]
- 10.Li Y, Xu Y, Zheng T, Wang H. Flocculation mechanism of the actinomycete Streptomyces sp. hsn06 on Chlorella vulgaris. Bioresource Technol. 2017;239:137–143. doi: 10.1016/j.biortech.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Alam MA, Vandamme D, Wan C, Zhao XQ, Foubert I, Wang ZM, Muylaert K, Yuan ZH. Bioflocculation as an innovative harvesting strategy for microalgae. Rev Environ Sci Bio. 2016;15:1–11. doi: 10.1007/s11157-016-9408-8. [DOI] [Google Scholar]
- 12.Pishgar Z, Samimi A, Mohebbi-Kalhori D, Shokrollahzadeh S. Comparative study on the harvesting of marine Chlorella vulgaris microalgae from a dilute slurry using autoflocculation-sedimentation and electrocoagulation-flotation methods. Int J Environ Res. 2020;14:615–628. doi: 10.1007/s41742-020-00277-y. [DOI] [Google Scholar]
- 13.Vu HP, Nguyen LN, Lesage G, Nghiem LD. Synergistic effect of dual flocculation between inorganic salts and chitosan on harvesting microalgae Chlorella vulgaris. Environ Technol Innov. 2020;17:100622. doi: 10.1016/j.eti.2020.100622. [DOI] [Google Scholar]
- 14.Kumaran J, Singh I, Joseph V. Effective biomass harvesting of marine diatom Chaetoceros muelleri by chitosan-induced flocculation, preservation of biomass, and recycling of culture medium for aquaculture feed application. J Appl Phycol. 2021;33:1605–1619. doi: 10.1007/s10811-021-02369-4. [DOI] [Google Scholar]
- 15.Loganathan K, Saththasivam J, Sarp S. Removal of microalgae from seawater using chitosan-alum/ferric chloride dual coagulations. Desalination. 2018;433:25–32. doi: 10.1016/j.desal.2018.01.012. [DOI] [Google Scholar]
- 16.Sanyano N, Chetpattananondh P, Chongkhong S. Coagulation–flocculation of marine Chlorella sp. for biodiesel production. Bioresour Technol. 2013;147:471–476. doi: 10.1016/j.biortech.2013.08.080. [DOI] [PubMed] [Google Scholar]
- 17.Pirwitz K, Rihko-Struckmann L, Kai S. Comparison of flocculation methods for harvesting Dunaliella. Bioresource Technol. 2015;196:145–152. doi: 10.1016/j.biortech.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Roselet F, Vandamme D, Muylaert K, Paulo CA (2019) Harvesting of microalgae for biomass production In: Alam M, Wang Z (ed) Microalgae biotechnology for development of biofuel and wastewater treatment. Springer, Singapore, pp 211–243. 10.1007/978-981-13-2264-8_10
- 19.Cheng YS, Zheng Y, Labavitch JM, Vandergheynst JS. The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella. Process Biochem. 2011;46:1927–1933. doi: 10.1016/j.procbio.2011.06.021. [DOI] [Google Scholar]
- 20.Zhu LD, Hu TY, Li SX, Nugroho YK, Li BS, Cao J, Show PL, Hiltunen E. Effects of operating parameters on algae Chlorella vulgaris biomass harvesting and lipid extraction using metal sulfates as flocculants. Biomass Bioenerg. 2020;132:105433. doi: 10.1016/j.biombioe.2019.105433. [DOI] [Google Scholar]
- 21.Nair AT, Ahammed MM. Coagulant recovery from water treatment plant sludge and reuse in post-treatment of UASB reactor effluent treating municipal wastewater. Environ Sci Pollut Res. 2014;21:10407–10418. doi: 10.1007/s11356-014-2900-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhu LD, Li ZH, Hiltunen E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol Biofuels. 2018;11:183. doi: 10.1186/s13068-018-1183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamshad A, Kothari R, Singh HM, Tyagi VV, Singh B, Sari A. Experimental investigation of microalgal harvesting with low cost bottom ash: Influence of temperature and pH with zeta potential and thermodynamic function. Environ Technol Innov. 2021;22:101376. doi: 10.1016/j.eti.2021.101376. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.