Fig. 4.
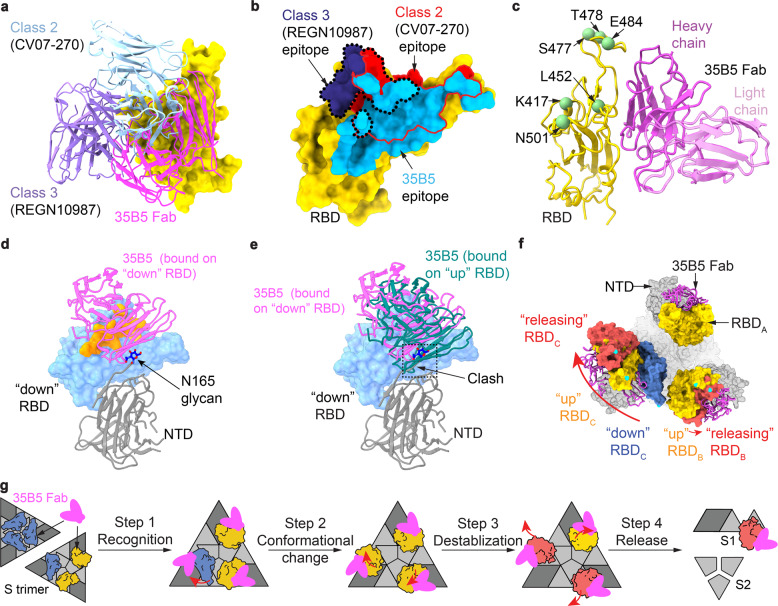
Structural basis and neutralizing mechanism for the broad neutralizing activities of 35B5 to the SARS-CoV-2 variants. a Structural comparisons of the 35B5 Fab- “up” RBD interactions with those of the previously identified Class 2 and 3 neutralizing antibodies. 35B5 Fab and the Fab regions of the representative antibodies CV07-270 and REGN10987 from Class 2 and 3, respectively, are illustrated in cartoon and colored as indicated. RBD is represented as surface in yellow. b Comparison of the epitope of 35B5 with those of the representative neutralizing antibodies of Class 2 and 3 on RBD. The 35B5 epitope is colored in cyan. The epitopes of CV07-270 (Class 2) and REGN10987 (Class 3) are labeled using red and black lines, respectively. c Mapping of the frequently mutated residues of the spike protein in the recent SARS-CoV-2 variant in the 35B5 Fab-RBD structure. RBD and 35B5 Fab are shown in cartoon and colored in yellow and purple, respectively. The frequently mutated residues in RBD in the recent SARS-CoV-2 variant are highlighted with green spheres. d Interactions of 35B5 Fab with the “down” RBD. 35B5 Fab, the “down” RBD, and NTD domains are colored in purple, cyan, and gray, respectively. The 35B5 Fab-interacting region on RBD is highlighted in orange. e Structural clashes between 35B5 Fab and the NTD domain upon structural superimposition of the “up” RBD-35B5 Fab model with the “down” RBD-35B5 Fab region in the state 1 S-6P-35B5 Fab complex. The structural clashes are highlighted with dashed lines. f Conformational changes of the RBD domains upon 35B5 Fab binding. The S-6P-35B5 Fab complex structures of three states were superimposed. 35B5 Fabs are shown in the cartoon in purple. The “down”, “up”, and “releasing” RBD domains are colored in blue, yellow, and red, respectively. The conformational changes of RBDs are indicated with arrows. g Schematic diagram of the neutralizing mechanism of 35B5 against SARS-CoV-2. The “down” and “up” RBDs in the tight-closed or loose-closed S trimers are colored in blue and yellow, respectively. The conformation changes and transmission of RBDs from the “down” to “up”, finally to “releasing” states are shown as red arrows