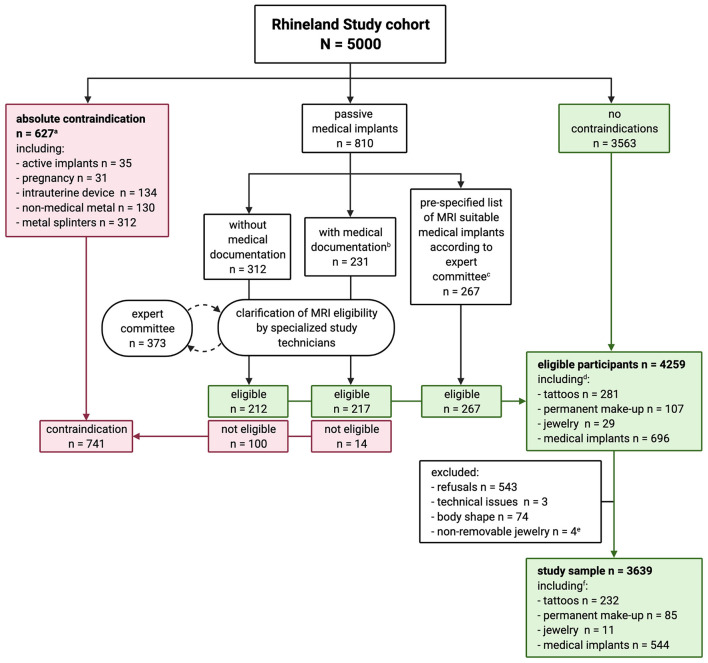
Figure 1.
Flowchart of the process of clarification of MRI suitability in the Rhineland Study. aParticipant could have more than one absolute contraindication. bOnly three participants had MRI safety certificates for their medical implants. cAfter evaluating our procedure after 1 year, the expert committee considered the following medical implants, if implanted after 2005, as MRI suitable without checking further documentation: hip and knee replacements, stents, bypass, breast implants filled with silicone, and screws, plates and stiffening of the spinal cord < 13 cm. dThree hundred and seventy-six participants had tattoos and/or permanent make-up, of whom 45 also had medical implants. eParticipants who were excluded according to stricter exclusion criteria at study start and could not be contacted for reinvitation. fThree hundred and five participants had tattoos and/or permanent make-up, of whom 35 also had medical implants.