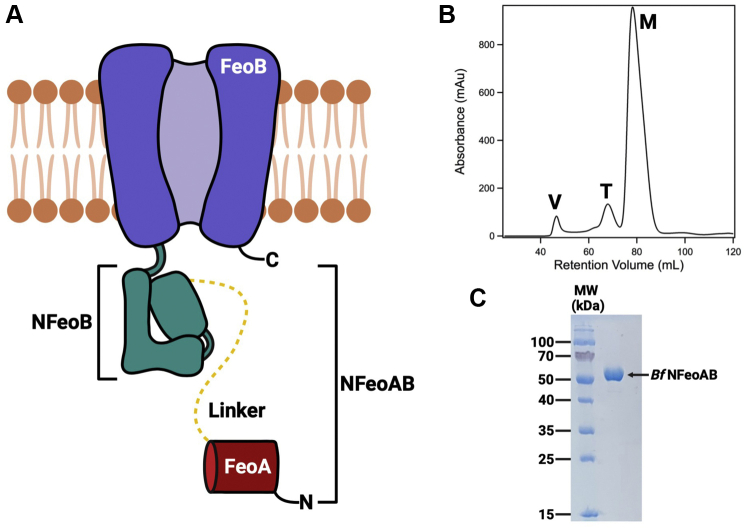
Figure 2.
Predicted domain topology of the BfNFeoAB construct used in this work and its purification.A, cartoon representation of the FeoAB fusion protein from Bacteroides fragilis. The FeoA protein (red) is covalently tethered to NFeoB (teal) through the G-protein domain by a predicted 44-amino acid linker region (dashed yellow line). The soluble NFeoAB domain is tethered to the transmembrane region of FeoB (purple). Labels ‘N’ and ‘C’ refer to the N- and C-termini, respectively. B, SEC purification of BfNFeoAB on a 120 ml Superdex 200 column. The majority of BfNFeoAB is monomeric (≈80 ml retention volume; ‘M’), while no more than 10% of BfNFeoAB is either trimeric (≈70 ml retention volume; ‘T’) or aggregated (≈45 ml retention volume; ‘V’). C, based on SDS-PAGE, BfNFeoAB is estimated to be >95% pure after SEC in panel B. Figure created with BioRender.