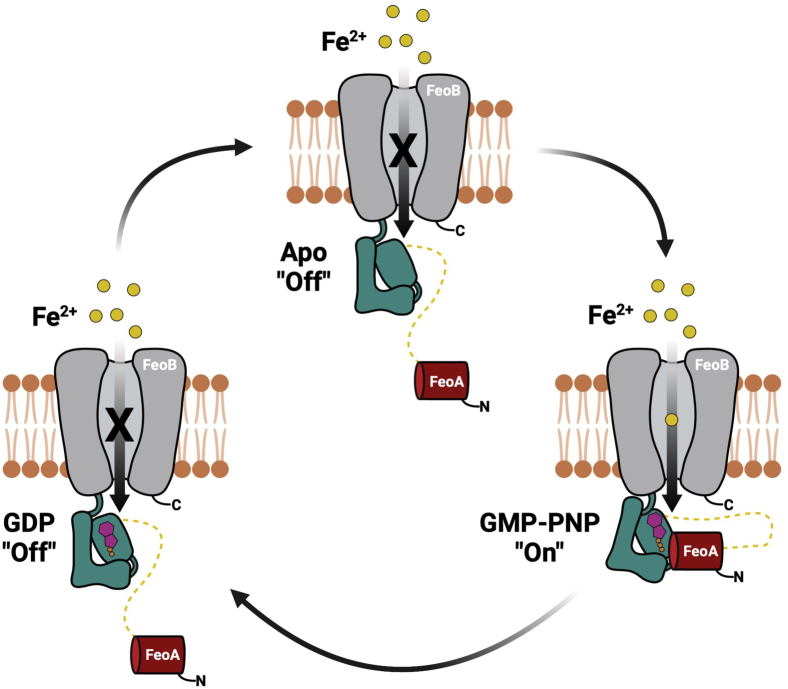
Figure 7.
A model of the proposed FeoA-based regulatory function on iron transport of BfFeoAB. In the absence of nucleotide (apo; top), FeoA (red) does not interact with NFeoB (teal), thus transport of Fe2+ (yellow) through the transmembrane domain (gray) is in an “off” state. Binding of GMP-PNP (magenta and orange; right), a GTP-analog, to NFeoB induces protein-protein interactions between NFeoB and FeoA. We posit that this GMP-PNP-bound state sends a signal to the transmembrane region to “turn on” Fe2+ transport. GDP binding to NFeoB (magenta and orange; left) transduces a signal the transmembrane domain to “turn off” Fe2+ transport before dissociation of FeoA-NFeoAB interactions, loss of GDP, and a return of the transporter to the apo state.