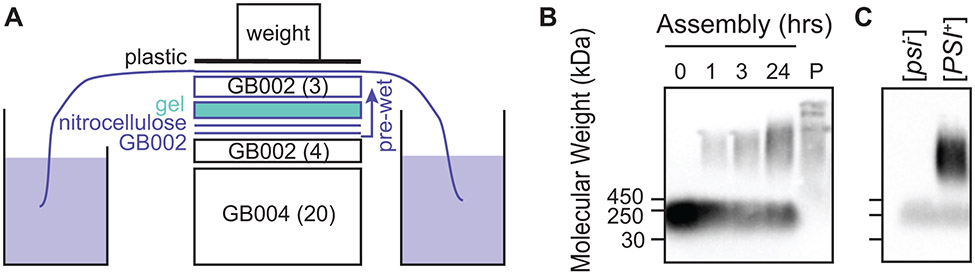
Figure 4:
Semi-Denaturing Detergent-Agarose Gel Electrophoresis (SDD-AGE) is used to visualize the aggregation state of recombinantly-expressed, exogenously-added proteins. After separation of large protein aggregates on agarose gels, the proteins are transferred to a nitrocellulose membrane using capillary transfer. Because the read-out is antibody based, SDD-AGE is appropriate for analysis of proteins at low concentrations in complex mixtures. A) Transfer of proteins to the nitrocellulose membrane is accomplished by capillary transfer. The agarose gel is placed in direct contact with a wet nitrocellulose membrane. The gel and membrane are placed on a stack of dry blotting paper, covered by wet blotting paper kept moist through the action of a wick in a reservoir of buffer. B) The kinetics of assembly of exogenously-added NM (visualized in this case by an antibody against a his6 tag) into the amyloid form in yeast lysates (e.g. Figure 2C). Monomeric NM is removed by centrifugation as evidenced by analysis of the pellet (P) as described in Figure 2D. C) Amyloid aggregates remain intact after the addition of cryoprotectants, polarization agents and freezing (e.g. Figure 2E). Moreover, these manipulations do not induce the formation of amyloid as evidenced by the lack of high molecular weight aggregates in samples prepared using [psi−] yeast.