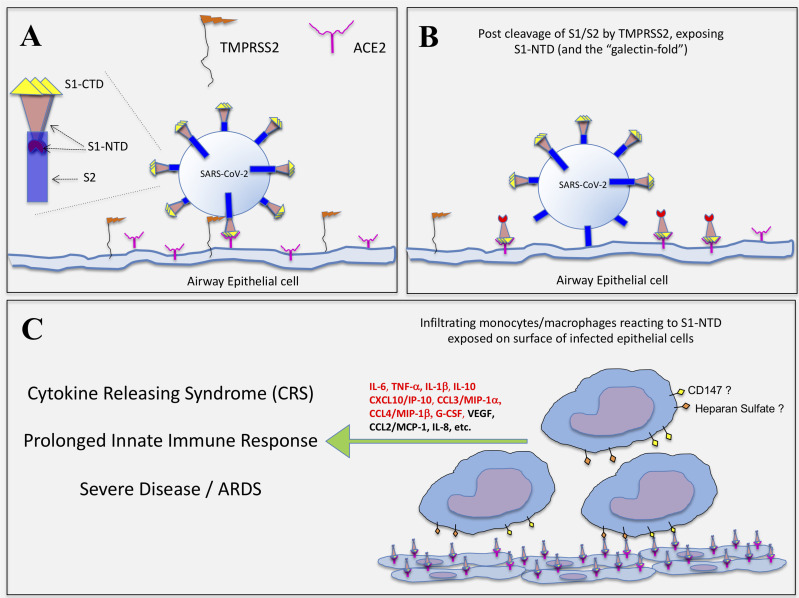
Figure 5.
Hypothetical representation of how SARS-CoV-2 infection exposes the S1-NTD (and “galectin-fold”) on epithelial cells for potential activation of infiltrating monocytes. (A) SARS-CoV-2 infection of epithelial cells is initiated with the binding of S1-CTD/RBD to ACE2. The serine protease, TMPRSS2, expressed on host cells then cleaves the spike protein at the S1/S2 linkage. (B) The S2 subunit undergoes structural changes, serving first to anchor the virus and then facilitating its entry into the cell. It is proposed that the S1/S2 cleavage event simultaneously exposes the S1-NTD, which extends outward as the S1-CTD/RBD remains bound to ACE2. (C) Infiltrating monocytes/macrophages are then activated to produce COVID-related cytokines via cell surface glycoproteins (e.g. CD147) and/or polysaccharides (e.g. Heparan Sulfate) interacting with S1-NTD, which mimics EC-Gal-3. Those cytokines indicated in red type were significantly impacted by the S1 subunit in this study.