FIGURE 1.
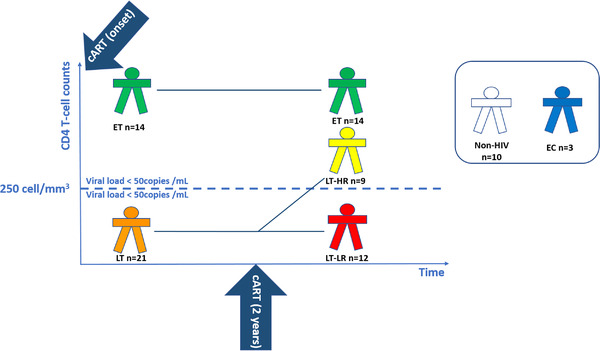
Schematic diagram showing the selection criteria and number of subjects enrolled in each study group. The following human immunodeficiency virus (HIV)‐treated groups (ET, LT‐HR and LT‐LR) were recruited according to immunological criteria: early‐treated (ET, n = 14), who started cART with >250 CD4 T cells/mm3 and remained >250 CD4 T cells/mm3 2 years later; late‐treated high recovery (LT‐HR, n = 9), starting last cART with <250 CD4 T cells/mm3 but reaching >250 CD4 T cells/mm3 2 years later; late‐treated low‐recovery (LT‐LR, n = 12), starting cART with <250 CD4 T cells/mm3 and remaining <250 CD4 T cells/mm3 2 years later. All these cART‐treated HIV‐infected subjects were virologically suppressed (<50 HIV RNA copies/ml). Additionally, a reduced group of elite controllers (EC, n = 3) was also included. EC are HIV‐subjects with spontaneous virological suppression in the absence of cART. Control healthy subjects (n = 10) were individuals with similar age and sex characteristics to those of treated HIV‐subjects. Blood and biopsy samples of ileum and caecum mucosa were taken during colonoscopy procedures made a variable time after compliance of 2 years of cART as the classification period. All subjects maintained their classification criteria at the moment of collection of samples. Exclusion criteria for this study were having HIV rebounds during the classification period and until the recruitment; intestinal infections, cancer, active hepatitis C virus (HCV) infection or concurrent inflammatory processes at the moment of recruitment. All the subjects enrolled in this study were properly informed and signed the corresponding informed consents either for the colonoscopy and the experimental study. ET, early‐treated; LT, late‐treated; LT‐HR, late‐treated high recovery; LT‐LR, late‐treated low recovery; EC, elite controllers; cART, combined antiretroviral therapy
