Figure 3. UBP13 interacts with and deubiquitinates BRI1.
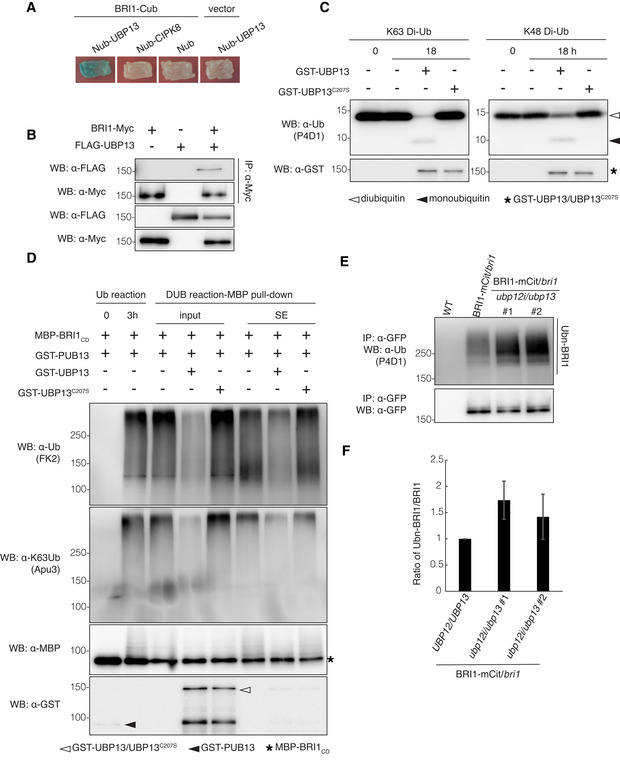
- Split‐ubiquitin yeast two‐hybrid assay revealing the UBP13 and BRI1 interactions. The indicated constructs were co‐transformed into yeast cells. BRI1‐Cub with Nub, Nub‐CIPK8, and Nub‐UBP13 with an empty vector were used as negative controls. Transformants were streaked onto solidified medium supplemented with X‐gal. Blue patch indicates positive interaction. Cub, the C‐terminal half of ubiquitin; Nub, the N‐terminal half of ubiquitin.
- UBP13 interaction with BRI1 in plants. BRI1‐Myc and FLAG‐UBP13 were co‐expressed in Nicotiana benthamiana leaves and co‐immunoprecipitation was done on solubilized microsomal proteins with α‐Myc antibody beads. The association of BRI1‐UBP13 was detected by western blot with an α‐FLAG antibody after α‐Myc IP (Top).
- Deubiquitinating activity of UBP13 against K48‐ and K63‐linked ubiquitination. Diubiquitins linked through K48 or K63 (K48 Di‐Ub or K63 Di‐Ub) were incubated alone or with GST‐UBP13 or GST‐UBP13C207S for 18 h. Deubiquitination of the diubiquitins was analyzed by western blot with the α‐ubiquitin (α‐Ub, P4D1) antibody. The presence of GST‐UBP13/UBP13C207S recombinant proteins was confirmed by the α‐GST antibody.
- UBP13 deubiquitination of K63‐ubiquitinated BRI1 in vitro. Polyubiquitinated MBP‐BRI1CD was generated by incubation with GST‐PUB13 (Ub reaction) and then with GST‐UBP13 or GST‐UBP13C207S (DUB reaction‐MBP pull‐down, input). After in vitro deubiquitination, MBP‐BRI1CD was purified by an MBP pull‐down to analyze the BRI1CD ubiquitination (DUB reaction‐MBP pull‐down, SE). The ubiquitinated proteins were detected by western blot with an α‐ubiquitin antibody (FK2). Ubiquitin chain specificity was detected with an α‐K63Ub antibody (Apu3). The presence of recombinant proteins was confirmed by the α‐GST and α‐MBP antibodies.
- Western blot analysis of BRI1 ubiquitination on plants expressing pBRI1:BRI1‐mCitrine complementing the bri1 mutant in either the UBP12/UBP13 (BRI1‐mCit/bri1) or ubp12i/ubp13 (BRI1‐mCit/bri1/ubp12i/ubp13) background. BRI1‐mCitrine proteins were isolated from 18‐day‐old plants grown on DEX medium and then immunoprecipitated with α‐GFP antibody beads from solubilized microsomal proteins. Ubiquitinated BRI1 and basal BRI1 proteins were detected by the α‐ubiquitin (P4D1) and α‐GFP antibodies, respectively.
- Quantification of BRI1 ubiquitination profiles from (E) (n = 3 biological replicates). The ubiquitinated BRI1 fraction was normalized to the total immunoprecipitated BRI1 detected by the α‐GFP antibody.
Data information: Data in (F) are presented as means ± SD.
